Note on Accessibility: Persons using mobile devices may find some tables are not fully accessible. Note that you can view tables on a larger screen or in the PDF version of the report/monograph. If you need additional assistance, email us or use our contact form and identify the tables for which access is required. We will assist you in accessing the content. NIEHS has helpful information on accessibility.
Developmental & Reproductive Toxicity Report 07
NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits

Abstract
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) is a juvenile hormone mimetic pesticide used to control a variety of insects, including tsetse flies, cockroaches, and whiteflies, and is added to potable water in Zika virus-endemic areas to control mosquitoes. It has been proposed that MPEP might contribute to the increased incidence of microcephaly in babies born to mothers who could be consuming MPEP in potable water. Because limited information is available about the potential hazard of MPEP to pregnant women, the National Toxicology Program (NTP) conducted prenatal developmental toxicity studies of MPEP to assess possible harm to the developing conceptus and pregnant animal. In these studies, time-mated Sprague Dawley (Hsd:Sprague Dawley® SD®) rats and New Zealand White (Hra:NZW SPF) rabbits were administered MPEP in corn oil by gavage once daily from implantation on gestation day (GD) 6 (rats) or GD 7 (rabbits) to the day before expected parturition (GD 20 for rats; GD 28 for rabbits). In the prenatal developmental toxicity study in rats, fetuses were examined for evidence of MPEP fetal toxicity. A dose range-finding study in rabbits was conducted, followed by a prenatal developmental toxicity study, to confirm the absence of a response in a second species. An assessment of maternal and fetal MPEP concentrations following exposure demonstrated maternal-fetal transfer of MPEP in both rats on GD 18 and rabbits on GD 28.
Prenatal Developmental Toxicity Study in Rats
Dose selection was informed by summary data provided for marketing approval, and an additional dose level was added to aid in dose-response characterization. Groups of 25 time-mated female rats were administered 0, 62.5, 125, 250, or 500 mg MPEP/kg body weight/day (mg/kg/day) in corn oil by gavage once daily from GD 6 to GD 20.
After initiation of dosing (GD 6–9), dams administered either 250 or 500 mg/kg/day displayed similar significantly decreased (~25%) mean body weight gains relative to vehicle control animals. This finding occurred concomitantly with significantly decreased (~11%) feed consumption in the 500 mg/kg/day group, demonstrating limited maternal toxicity. Exposure to MPEP did not affect any pregnancy or litter parameters. Fetal weight in the 500 mg/kg/day group was slightly lower (<4%), with a significant trend, and was not associated with an increased incidence of ossification variants.
Fetal visceral findings included small increases in the incidences of liver discoloration (a variation), which could have been incidental but may be indirect effects of MPEP on fetal liver function or metabolism. No external, visceral, head, or skeletal malformations were attributed to MPEP exposure.
Dose Range-finding Study in Rabbits
Groups of eight time-mated female rabbits were administered 0, 300, 400, or 500 mg/kg/day MPEP in corn oil by gavage once daily from GD 7 to GD 28. Information from previous work indicated a likely sharp dose-response curve for maternal toxicity. Decreased feed consumption and decreases in mean body weight indicative of overt maternal toxicity were observed at doses of 400 and 500 mg/kg/day, resulting in those dose groups being removed from the study and fetuses not examined. Similar, but less severe, maternal findings were noted at 300 mg/kg/day. Uterine and fetal weights were slightly lower in the 300 mg/kg/day dose group relative to the vehicle control group, and these findings may have been secondary to maternal toxicity. No external or placental observations were attributed to MPEP exposure. A high dose of 250 mg/kg/day was therefore selected for the prenatal developmental toxicity study.
Prenatal Developmental Toxicity Study in Rabbits
Time-mated rabbits (23 or 24 per dose group) were administered 0, 62.5, 125, or 250 mg/kg/day of MPEP in corn oil by gavage once daily from GD 7 to GD 28. An additional 3 or 4 does per dose group, used for biological sampling, were administered the same doses of MPEP. The 250 mg/kg/day dose was generally well tolerated by most does; however, decreases in feed consumption, mean body weight, and body weight gain were observed in this dose group, resulting in three animals being removed early from the study. These early removals, in addition to two nonpregnant does and two does that underwent parturition prior to laparotomy, collectively resulted in 16 litters in the 250 mg/kg/day group available for examination. Litter size, postimplantation loss, and fetal weight were not affected by MPEP exposure, demonstrating that although the 250 mg/kg/day dose resulted in some maternal toxicity, the does and fetuses received the highest dose possible without overt impact on maternal function that may impact fetal outcomes.
No external, visceral, or head malformations were attributed to MPEP exposure. A single incidence of hydrocephaly was noted in one fetus in the 125 mg/kg/day group, but this finding was considered incidental. Three fetuses from two litters and three fetuses from one litter in the 125 and 250 mg/kg/day dose groups, respectively, displayed increased incidences of a skeletal malformation: seventh costal cartilage not fused to sternum. This structure is recognized by the International Federation of Teratology Societies but has not specifically been reported in the historical control data of commercial contract laboratories used for animal sourcing. The absence of this cartilaginous structure could result from a delay in development independent of maternal toxicity, given that individual doe mean body weight gains and feed consumption were similar to those of does whose fetuses were not observed with this malformation.
Exposure to MPEP was confirmed in pregnant rats and rabbits and MPEP was detected in the fetuses, demonstrating that fetuses were exposed to MPEP.
Conclusions
Under the conditions of the rat prenatal developmental toxicity study, there was no evidence of developmental toxicity of 2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) in Sprague Dawley (Hsd:Sprague Dawley® SD®) rats administered 62.5, 125, 250, or 500 mg/kg/day based on the absence of effects on reproductive parameters, fetal weight, or increased incidence of fetal malformations or variations. The highest dose administered was 500 mg/kg/day, which did not result in overt maternal toxicity.
Under the conditions of the rabbit prenatal developmental toxicity study, there was equivocal evidence of developmental toxicity of MPEP in New Zealand White (Hra:NZW SPF) rabbits based on the occurrence of the malformation “seventh costal cartilage not fused to sternum” in dosed groups. This finding was observed at 250 mg/kg/day, a dose that induced some maternal toxicity.
Synonyms: MPEP; MPPE; pyridine, 2-[1-methyl-2-(4-phenoxyphenoxy)ethoxy]-; 2-[1-methyl-2-(4-phenoxyphenoxy)ethoxy]pyridine; 2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine; pyriproxyfen
Trade names: Nylar
Summary of Exposure-related Findings in Rats in the Prenatal Developmental Toxicity Gavage Study of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| 0 mg/kg/day | 62.5 mg/kg/day | 125 mg/kg/day | 250 mg/kg/day | 500 mg/kg/day | |
|---|---|---|---|---|---|
| Maternal parameters | |||||
| Animals on study | 25 | 25 | 25 | 25 | 25 |
| Number pregnant | 23 | 20 | 22 | 20 | 23 |
| Number found dead | 0 | 0 | 1 | 0 | 0 |
| Number removed – delivery before necropsy | 0 | 0 | 0 | 0 | 1 |
| Clinical observations | None | None | None | None | None |
| Body weight and feed consumptiona,b | |||||
| Necropsy body weight | 387.8 ± 5.1 | 390.3 ± 4.1 | 377.7 ± 8.0 | 379.8 ± 6.0 | 386.5 ± 5.4 |
| Body weight change GD 6–21 | 145.6 ± 4.1 | 147.2 ± 3.1 | 137.2 ± 7.5 | 137.0 ± 5.6 | 145.0 ± 4.3 |
| Feed consumption GD 6–21 | 21.7 ± 0.3 | 22.0 ± 0.3 | 21.5 ± 0.3 | 21.8 ± 0.4 | 22.2 ± 0.4 |
| Necropsy observations | None | None | None | None | None |
| Developmental/fetal parameters | |||||
| Number of litters examined | 23 | 20 | 22 | 20 | 22 |
| Number of live fetuses evaluated | 314 | 286 | 290 | 265 | 318 |
| Number of live fetuses per litterb | 13.65 ± 0.68 | 14.30 ± 0.55 | 13.81 ± 0.74 | 13.25 ± 0.73 | 14.45 ± 0.58 |
| Number of early resorptionsc | 23 | 9 | 9 | 11 | 8 |
| Number of late resorptionsc | 1 | 1 | 0 | 1 | 2 |
| Number of dead fetusesc | 0 | 1 | 0 | 0 | 0 |
| Number with whole litter resorptionsc | 0 | 0 | 1 | 0 | 0 |
| Percent postimplantation lossb | 6.90 ± 1.74 | 3.80 ± 1.09 | 8.58 ± 4.91 | 4.22 ± 1.10 | 3.10 ± 1.11 |
| Fetal body weight per littera,b | 5.35 ± 0.05* | 5.33 ± 0.05 | 5.43 ± 0.13 | 5.26 ± 0.06 | 5.17 ± 0.06 |
| Male fetal weight per littera,b | 5.47 ± 0.06* | 5.47 ± 0.05 | 5.58 ± 0.12 | 5.39 ± 0.06 | 5.29 ± 0.06 |
| Female fetal weight per littera,b | 5.21 ± 0.05* | 5.19 ± 0.06 | 5.17 ± 0.04 | 5.13 ± 0.06 | 5.02 ± 0.06* |
| Gravid uterine weighta,b | 98.91 ± 4.44 | 103.39 ± 3.26 | 99.80 ± 4.97 | 94.33 ± 4.80 | 102.13 ± 3.75 |
| External findings | None | None | None | None | None |
| Visceral findingsd | |||||
| Abdominal viscera | |||||
| Liver lobe, discolored – [V] | |||||
| Fetuses | 0 (0.00) | 0 (0.00) | 1 (0.34) | 4 (1.51) | 3 (0.94) |
| Litters | 0 (0.00) | 0 (0.00) | 1 (4.76) | 3 (15.00) | 2 (9.09) |
| Skeletal findings | None | None | None | None | None |
| MPEP concentrations (GD 18)b | |||||
| Dam plasma (ng/mL) | 3.5 ± 2.0 (3)e | 4,807.5 ± 835.7* (4) | –f | 5,406.7 ± 3,783.3 (3) | – |
| Amniotic fluid (ng/mL) | BD | 123.0 ± 23.9 (4) | – | 163.1 ± 40.8 (3) | – |
| Pooled fetal (ng/g) | 50.5 ± 19.5* (3) | 862.8 ± 119.5 (4) | – | 1,418.3 ± 665.7 (3) | – |
| Level of evidence of developmental toxicity: | No Evidence | ||||
Summary of Exposure-related Findings in Rabbits in the Prenatal Developmental Toxicity Gavage Study of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| 0 mg/kg/day | 62.5 mg/kg/day | 125 mg/kg/day | 250 mg/kg/day | |
|---|---|---|---|---|
| Maternal parameters | ||||
| Animals on study | 24 | 24 | 24 | 23 |
| Number pregnant | 24 | 23 | 24 | 21 |
| Number euthanized moribund | 0 | 0 | 1 | 3 |
| Number euthanized – early delivery | 0 | 0 | 0 | 1 |
| Number removed – delivery before necropsy | 0 | 0 | 1 | 1 |
| Clinical observations | None | None | None | None |
| Body weight and feed consumptiona,b | ||||
| Necropsy body weight | 3,383.4 ± 45.3 | 3,461.5 ± 42.3 | 3,399.5 ± 48.7 | 3,297.9 ± 65.5 |
| Body weight change GD 7–29 | 351.3 ± 27.8 | 431.5 ± 20.9 | 339.6 ± 28.8 | 282.0 ± 51.9 |
| Feed consumption GD 7–29 | 117.0 ± 3.7 | 133.1 ± 3.5** | 119.6 ± 3.4 | 116.6 ± 5.2 |
| Necropsy observations | None | None | None | None |
| Developmental/fetal parameters | ||||
| Number of litters examined | 24** | 23 | 22 | 16* |
| Number of live fetuses evaluated | 214 | 185 | 193 | 136 |
| Number of live fetuses per litterb | 8.92 ± 0.39 | 8.04 ± 0.41 | 8.77 ± 0.39 | 8.50 ± 0.43 |
| Number of early resorptionsc | 4 | 6 | 4 | 1 |
| Number of late resorptionsc | 2 | 3 | 1 | 2 |
| Number of dead fetusesc | 1 | 0 | 0 | 0 |
| Number with whole litter resorptionsc | 0 | 0 | 0 | 0 |
| Percent postimplantation lossb | 3.81 ± 1.36 | 4.83 ± 1.70 | 2.89 ± 1.17 | 2.23 ± 1.26 |
| Fetal body weight per littera,b | 37.98 ± 1.25 | 39.84 ± 0.97 | 37.07 ± 0.92 | 36.70 ± 1.37 |
| Male fetal weight per littera,b | 39.03 ± 1.47 | 40.52 ± 1.22 | 36.96 ± 1.00 | 37.41 ± 1.43 |
| Female fetal weight per littera,b | 36.65 ± 1.26 | 39.05 ± 0.96 | 37.25 ± 0.92 | 35.64 ± 1.57 |
| Gravid uterine weighta,b | 491.97 ± 15.51 | 471.69 ± 19.67 | 473.39 ± 17.01 | 461.36 ± 22.03 |
| External findings | None | None | None | None |
| Visceral findings | None | None | None | None |
| Skeletal findingsd | ||||
| Ribs | ||||
| Costal cartilage, 7th unilateral or bilateral, not fused to sternum – [M] | ||||
| Fetuses | 0 (0.0) | 0 (0.0) | 3 (1.58) | 3 (2.21) |
| Litters | 0 (0.0) | 0 (0.0) | 2 (9.09) | 1 (6.25) |
| MPEP concentrations (GD 28; 2 hours postdose)b | ||||
| Doe plasma (ng/mL) | 10.9 ± 0.5 (3)e | 52.6 ± 14.1 (3) | 223.2 ± 74.5 (3) | 206.0 ± 46.0 (3) |
| Pooled fetal plasma (ng/mL) | 9.7 ± 0.6 (3) | 66.1 ± 6.1 (3) | 158.0 ± 21.5 (3) | 255.0 ± 57.6 (3) |
| Level of evidence of developmental toxicity: | Equivocal Evidence | |||
Introduction
Chemical and Physical Properties
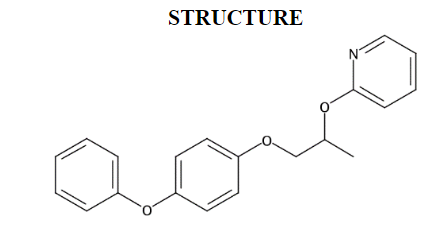
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP)1 is a solid with a molecular mass of 321.4 g/mol (Figure 1). Its appearance at technical grade has been reported as colorless crystalline, white to off-white and powdery, or pale yellow and waxy. It has a melting point of 47°C, a vapor pressure of <9.8 × 10−8 mm Hg at 23°C, and a log KOW of 5.37. MPEP has an estimated water solubility of 0.681 mg/L at 25°C and is readily soluble in hexane, methanol, and xylene.1
Production, Use, and Human Exposure
MPEP is an insecticide that acts as a juvenile insect hormone analog and growth regulator. Insect exposure to MPEP prevents larvae from developing into adulthood, thus rendering them unable to reproduce. MPEP can be formulated into granules, aerosols, baits, carpet powders, foggers, pet shampoos, and collars, as well as into a general surface spray for food and nonfood areas. MPEP is used in agriculture and applied to pasture grass and rangeland to control a variety of insect populations.2,3
MPEP is also added to potable water4 to control mosquito populations, including the species that carry Zika virus. The Zika virus, a member of the Flaviviridae family (which also includes yellow fever, dengue, West Nile, and Chikungunya viruses), is carried by Aedes mosquitoes (A. aegypti and A. albopictus) and can also be transmitted from human to human via body fluids.5 Initial infection in humans is often characterized by fever, rash, joint pain, and conjunctivitis, and pregnant women who are infected with Zika virus have an increased risk of giving birth to a baby with microcephaly and other abnormalities.6 An increase in microcephaly was not reported in previous Zika virus outbreaks, but an outbreak in Brazil in 2015 was associated with almost 4,000 microcephaly cases by 2016.5 The Zika virus has been reported to be present in fetal brain tissue, which is common with other viruses. An increased incidence of Guillain-Barré syndrome (inflammatory peripheral neuropathy) has also been observed in the adult population exposed to the Zika virus.5 Although several risk assessments4,7 of MPEP have indicated that likely exposure levels are safe, the Brazilian Association for Collective Health requested that all growth inhibitor insecticides not be used in potable water. This position was highlighted by a report from physicians in the crop-sprayed villages, as well as by others,8-10 suggesting an association between the increase in microcephaly and MPEP exposure.
Regulatory Status
The U.S. Environmental Protection Agency (EPA) has established tolerance levels ranging from 0.1 to 10 ppm for MPEP on specific fresh and processed vegetables and fruits, milk, and eggs with levels ≤20 ppm in citrus oil and 100 ppm in dried herbs.2 MPEP is also labeled for insect control on cotton, pasture grass, and rangeland.3 Adding MPEP granules to potable water in cisterns/barrels was approved by the World Health Organization in 2008 to control mosquito populations.11
Absorption, Distribution, Metabolism, and Excretion
Experimental Animals
Following a single oral administration of 2 or 1,000 mg/kg [14C]-labeled MPEP in male and female Sprague Dawley rats, the administered radioactivity was recovered in the feces (89%–92%) and urine (~8%) within 7 days of administration, although most of the radioactivity was excreted within 2 days.12 Radioactivity was distributed to tissues with peak concentrations in blood, kidney, and liver occurring 4–8 hours after, and in fat occurring 12–14 hours after, a single administration. On day 7 after a single oral dose, the residual radioactivity in tissues was <0.3% with fat containing the highest concentration. Following administration of a 2 mg/kg dose of [14C]-labeled MPEP, approximately 34% of the radioactivity was recovered in bile, suggesting part of the dose excreted in feces was likely from the absorbed dose. [14C]-labeled metabolites identified in feces, urine, and bile were products of hydroxylation at 2- or 4-positions of the terminal phenyl ring, products of hydroxylation at the 5-position of the pyridyl ring, and products from cleavage of the ether linkages and sulfation of the resulting phenols. The parent compound was detected only in feces, accounting for 25%–37% of the administered radioactivity.12
In liver microsomes, in the presence of nicotinamide adenine dinucleotide phosphate, most of the major metabolites of MPEP observed in vivo in rodents were also observed.13 Although no sex-related differences were observed in mouse microsomes, there were significant sex-related differences in rats for some of the metabolic pathways.
Humans
The literature contains no studies on the absorption, distribution, metabolism, or excretion of MPEP in humans.
Developmental and Reproductive Toxicity
Experimental Animals
Publicly available data on the developmental and reproductive toxicity of MPEP are limited and include two summaries of studies in rats (Slc:SD and CRL:SD) conducted by the manufacturer to support registration. The first study showed that in a combined pre- and postnatal study design, female Slc:SD rats administered 0, 100, 300, or 1,000 mg MPEP/kg body weight/day (mg/kg/day) in corn oil from gestation day (GD) 7 to GD 17 exhibited excessive toxicity (mortality) at 1,000 mg/kg/day, whereas a 15% decrease in body weight gain was observed at 300 mg/kg/day. Increased incidences of cervical vertebrae variations were observed in fetuses in the 300 and 1,000 mg/kg/day groups. Postnatal findings on postnatal day (PND) 21 and/or PND 56 in both exposed groups included dilation of the renal pelvis. No MPEP-related effects were noted on motor coordination, learning, or physical and emotional development. No MPEP-related effects on reproductive performance of the dams were observed.14
In the second multigenerational study, CRL:SD rats were exposed to 0, 200, 1,000, or 5,000 ppm MPEP in the diet; estimated chemical intake was 87 and 453 mg/kg/day for the 1,000 and 5,000 ppm groups, respectively. Decreases in body weight, body weight gain, and feed consumption were observed in both sexes and generations. An increase in liver weights was observed in both sexes and was associated with liver and kidney histopathological findings in F1 males.15
In a rabbit developmental toxicity study,16 melted and cooled MPEP was administered via gavage (~1 mL/kg) at doses of 0, 100, 300, or 1,000 mg/kg/day from GD 6 to GD 18. Mortality and moribundity were noted in rabbits in the 1,000 mg/kg/day dose group. Other findings at this dose included soft stools, decreased feed consumption, decreased activity and bradypnea, and premature delivery/abortions. Similar findings, at lower incidences, were also observed in the 300 mg/kg/day dose group. Fetuses could be assessed in only 4 of the 13 available pregnant rabbits in the 1,000 mg/kg/day dose group. The other dose groups had 13/14 (0 mg/kg/day), 12/12 (100 mg/kg/day), and 11/14 (300 mg/kg/day) litters examined from their respective pregnant rabbits. No exposure-related findings were observed in the four litters examined in the 1,000 mg/kg/day dose group.
Humans
The literature contains no studies on the developmental or reproductive toxicity of MPEP in humans.
General Toxicity
Experimental Animals
The rat oral median lethal dose (LD50) of technical grade MPEP is >5,000 mg/kg, and the rat dermal LD50 of MPEP is >2,000 mg/kg. The rat inhalation median lethal concentration (LC50) is >1.3 mg/L (highest concentration attainable). MPEP was not a dermal irritant or sensitizer in guinea pigs.17-22
Subchronic exposure of rats to MPEP via the diet at approximately 118–141 mg/kg/day was associated with higher mean total cholesterol and phospholipids, lower red blood cell, hematocrit, and hemoglobin counts, and higher relative liver weights compared to the control group (the no-observed-adverse-effect level was ~23.5–27.7 mg/kg/day). Subchronic exposure of dogs to MPEP via the diet at approximately 300 mg/kg/day and higher was associated with higher absolute and relative liver weights in males and higher incidences of hepatocellular hypertrophy in females compared to the control groups. These findings potentially represented adaptive changes. MPEP was not found to increase the incidences of neoplasms in either Sprague Dawley rats or CD-1 mice at a dietary exposure concentration of 3,000 ppm. Topical application of MPEP to rats at the limit dose of 1 g/kg was not associated with any adverse findings.23-27
Humans
The literature contains no studies on the general toxicity of MPEP in humans.
Study Rationale
Due to the concern that MPEP exposure may lead to increased incidences of birth defects in Zika virus-infected humans in regions where the insecticide is applied and because publicly available information is limited, NTP conducted studies to assess the potential for MPEP to induce fetal toxicity in well-characterized rat and rabbit prenatal developmental toxicity model systems. An assessment of blood and fetal concentrations was also included. In addition, MPEP was screened in a developmental zebrafish model.
Materials and Methods
Overview of Prenatal Developmental Toxicity Study Designs
Prenatal developmental toxicity studies are conducted to ascertain if in utero exposure to a test agent results in embryo-fetal death, structural malformations/variations, growth retardation, or functional deficits that are not secondary to overt maternal toxicity. Overt maternal toxicity has been shown to affect normal embryo-fetal growth and development (e.g., excessively lower maternal body weight gains and lower fetal weights, increased maternal stress in mice, and cleft palate).28-30 The presence of maternal toxicity, however, should not negate a priori an apparent fetal response. Rather, given the maternal/embryo-fetal interrelationship, fetal findings should be interpreted considering the maternal responses. Pregnant animals should be administered dose levels of test agent to the extent feasible (or limit dose) to obtain maximal dam and fetal exposure, thereby sufficiently challenging the test system to identify potential developmental hazards.31
The conduct of a dose range-finding study aids in the determination of dose selection when the potential for test agent-induced maternal toxicity is unknown and can provide preliminary information on embryo-fetal outcomes (e.g., postimplantation loss, changes in fetal weight, external defects), and informs the prenatal developmental toxicity study design. In the prenatal developmental toxicity study, fetal examination is expanded to include examination of the fetal viscera, head (soft tissue and skeletal components), and the skeleton for osseous and cartilaginous defects. Abnormalities are separated into malformations that are permanent structural changes that might adversely affect survival, development, or function or variations that are a divergence beyond the usual range of structural constitution that might not adversely affect survival or health,29 consistent with that described by Makris et al.32 The study design for the prenatal developmental toxicity study in rats is presented in Figure 2, and the study design for the dose range-finding and prenatal developmental toxicity studies in rabbits is presented in Figure 3.
Procurement and Characterization
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) was obtained from AK Scientific, Inc. (Union City, CA) in a single lot (JL44164). Identity, purity, and stability analyses were conducted by the analytical chemistry lab at RTI International (Research Triangle Park, NC) (Appendix A). Reports on analyses performed in support of the MPEP studies are on file at the National Institute of Environmental Health Sciences (NIEHS).
MPEP was received as a white powder. The melting point was consistent with the literature values. Elemental analysis of lot JL44164 was consistent with the composition of MPEP. The identity of lot JL44164 was confirmed using infrared spectroscopy, 1H nuclear magnetic resonance (NMR) spectroscopy, 13C NMR spectroscopy, and three types of 2-dimensional NMR spectroscopy. The spectra were consistent with the reference and predicted spectra and with the structure of MPEP. Gas chromatography (GC) with mass spectrometry detection (MS) also confirmed the structure of MPEP.
Karl Fisher titration determined the water content of lot JL44164 to be 0.03%. The purity estimated by GC with flame ionization detection (FID) and by ultra-performance liquid chromatography (UPLC) with a photodiode array detector (PDA) was 99.6% and 97.7%, respectively. The UPLC/PDA analysis identified two impurity peaks with >0.1% and 15 additional peaks with <0.1% of the total response. The overall purity of lot JL44164 was estimated to be >97.7%.
Accelerated stability studies confirmed that lot JL44164 was stable for at least 2 weeks when stored in amber glass bottles sealed with Teflon-lined caps at 25, 5, and −20°C. Lot JL44164 was homogenized by stirring, distributed into 80-ounce bottles with Teflon-lined caps, and stored at room temperature.
Corn Oil
Corn oil was obtained from Welch, Holme & Clark Co. Inc (Newark, NJ) in a single lot (0120-0576) and used as a vehicle in the dose range-finding and prenatal developmental toxicity studies. A solubility and suspendability study of MPEP in corn oil determined that the test article was suspendable at up to 250 mg/mL and soluble at up to 136 mg/mL. Lot 0120-0576 contained peroxide levels that were less than the rejection level of 3 milliequivalents (meQ)/kg corn oil.
Preparation and Analysis of Dose Formulations
Dose formulations of MPEP were prepared in corn oil following the protocols outlined in Table A-2. The rat and rabbit prenatal developmental toxicity studies used dose formulations of 31.25, 62.5, 125, and 250 mg/mL (rat only). Dose formulations of 150, 200, and 250 mg/mL were used in the rabbit dose range-finding study. A homogeneity study at 250 mg/mL and a stability study at 1 mg/mL dose formulations were conducted using GC/FID. Homogeneity and stability were confirmed for 42 days at room temperature (~25°C).
Analysis of preadministration and postadministration dose formulations was conducted using GC/FID (Table A-3, Table A-4, Table A-5). All formulations were within 10% of the target concentrations except the 250 mg/mL postadministration sample in the rabbit dose range-finding study which was 21.3% above the target concentration.
Animal Source
Female Sprague Dawley (Hsd:Sprague Dawley® SD®) rats for use in the prenatal developmental toxicity study were obtained from Envigo (formerly Harlan Laboratories, Inc., Indianapolis, IN). Sexually mature (11 to 13 weeks old) females were time-mated overnight at the vendor and were received on gestation day (GD) 1 or GD 2. GD 0 was defined as the day positive evidence of mating was observed.
Female New Zealand White (Hra:NZW SPF) rabbits for use in the dose range-finding and prenatal developmental toxicity studies were obtained from Covance Research Products (Denver, PA). Sexually mature females (6 months old) were time-mated at the vendor and were received on GD 1 or GD 2.
Animal Welfare
Animal care and use are in accordance with the Public Health Service Policy on Humane Care and Use of Animals. All animal studies were conducted in an animal facility accredited by AAALAC International. Studies were approved by the Southern Research Animal Care and Use Committee and conducted in accordance with all relevant National Institutes of Health (NIH) and National Toxicology Program (NTP) animal care and use policies and applicable federal, state, and local regulations and guidelines.
Animal Health Surveillance
Prenatal Developmental Toxicity Study in Rats
Ten nonmated female rats were received for use as sentinels. Within 5–10 days of arrival and at study termination, blood was collected from the retroorbital plexus of sentinel animals for disease screening. After blood collection, animals were euthanized, necropsied, and examined for internal and external parasites. Necropsy included macroscopic examination of the external surface of the body; all orifices; cranial, thoracic, and abdominal cavities and their contents; and organs and tissues. All lesions were retained, and a histopathological examination was performed.
Serum was prepared from collected blood samples, diluted in saline, and stored frozen until shipped to the NTP-designated disease screening contract laboratory (IDEXX BioResearch, Columbia, MO) for analysis. Sera were analyzed for the presence of pathogens according to the protocols of the NTP Sentinel Animal Program (Appendix C). All test results were negative (Table C-1).
Dose Range-finding Study in Rabbits
Disease screening was performed on nine randomly selected time-mated does across dose groups. Blood samples were collected from all animals at study termination using Opti Spot, allowed to dry, and stored at ambient temperature until shipped to IDEXX BioResearch (Columbia, MO). Opti Spot samples were also collected from four additional does that were euthanized moribund prior to GD 29. Fecal, fur, and oral swab samples were collected from the nine randomly selected does at study termination, stored at ambient temperature, and shipped to IDEXX BioResearch (Columbia, MO). After sample collection, animals were given a gross necropsy and evaluated for external and internal parasites including ectoparasite and endoparasite screening.
Details on the serology tests performed are presented in Appendix C. Antibodies to rotavirus were detected in all samples; all other test results were negative (Table C-2).
Prenatal Developmental Toxicity Study in Rabbits
Disease screening was performed in 10 time-mated does randomly selected from vehicle control group animals. Blood samples were collected from one animal at the start of the study and all animals at study termination using Opti Spot, allowed to dry, and stored at ambient temperature until shipped to IDEXX BioResearch (Columbia, MO). Fecal samples and fur swabs were collected from the same does at study termination, stored at ambient temperature, and shipped to IDEXX BioResearch (Columbia, MO). After sample collection, animals were euthanized, necropsied, and examined for internal parasites. Gross lesions were retained for potential histopathological evaluation.
Serum samples were analyzed for the presence of pathogens according to the protocols of the NTP Sentinel Animal Program (Appendix C). Antibodies to rotavirus were detected in all samples that were tested; all other test results were negative (Table C-2).
Experimental Design
In the rat prenatal developmental toxicity study, time-mated animals were housed individually, provided NIH-07 feed and water ad libitum, and observed at least twice daily for viability (morning and afternoon, with at least 6 hours between observations). Clinical observations were recorded on arrival, on GD 3, and daily during dosing (GD 6–20) until removal for necropsy (1–3 hours after dosing). Dams were weighed on arrival, on GD 3, and daily from GD 6 through GD 21. Feed consumption was recorded at 3-day intervals: GD 3–6, GD 6–9, GD 9–12, GD 12–15, GD 15–18, and GD 18–21. Details of the study design, including animal source and identification, diet, water, husbandry, environmental conditions, euthanasia, necropsy, and fetal evaluations, are summarized in Table 1. Information on feed composition and contaminants is provided in Appendix B.
In the rabbit dose range-finding and prenatal developmental toxicity studies, time-mated animals were housed individually, provided Purina 5L3M feed and water ad libitum, and observed at least twice daily for viability (morning and afternoon). Clinical observations were recorded on arrival and daily during dosing (GD 7–28) until removal (1–3 hours after dosing). Does were weighed on arrival and daily from GD 3 through GD 29. Feed consumption was recorded daily from GD 3 through GD 29. Details of the rabbit study design, including animal source and identification, diet, water, husbandry, environmental conditions, euthanasia, necropsy, and fetal evaluations, are summarized in Table 2. Information on feed composition and contaminants is provided in Appendix B.
On GD 21, dams in the rat prenatal developmental toxicity study were weighed, euthanized with carbon dioxide, and examined for gross lesions of the thoracic and abdominal cavities. On GD 29, does in both the rabbit dose range-finding and prenatal developmental toxicity studies were weighed, euthanized with intravenous injection of sodium pentobarbital-containing solution, and examined for gross lesions of the thoracic and abdominal cavities, including the stomach for trichobezoars. The gravid uterus and ovaries were excised and weighed, and any placental findings were recorded. The numbers of uterine implantation sites and corpora lutea visible on the surface of each ovary were recorded. Uterine contents were examined for pregnancy status, and the numbers and locations of all live and dead fetuses (a live fetus is defined as one that responds to stimuli; a dead fetus is defined as a term fetus that does not respond to stimuli and is not markedly autolyzed) and resorptions were recorded.
Resorptions were classified as early or late. Early resorptions included a conceptus characterized by a grossly necrotic mass that had no recognizable fetal form and presence of nidation sites (“pregnant by stain”). Late resorptions were characterized by grossly necrotic but recognizable fetal form with placental remnants visible.33,34 Postimplantation loss was calculated as the number of dead and resorbed conceptuses divided by the total number of implantations (multiplied by 100). For each uterus with no macroscopic evidence of implantation, the uterus was stained with 10% (v/v) ammonium sulfide to visualize any possible implantation sites.35
Adult female rats that were euthanized moribund, delivered early, or found dead received a gross necropsy that included an examination of the thoracic and abdominal viscera for evidence of dosing trauma or toxicity. The uterus of each female was examined and stained, if necessary, to determine pregnancy status. Dams were not retained for further examination.
All female rabbits that aborted (defined as delivering before GD 29), were euthanized moribund, or found dead received a gross necropsy that included examination of the thoracic and abdominal viscera for evidence of dosing trauma, toxicity, and gross lesions. The uterus of each female was examined and stained, as necessary, to determine pregnancy status. Does were not retained for further examination.
Dose Selection Rationale for the Prenatal Developmental Toxicity Study in Rats
Dose selection was made on the basis of the sponsoring manufacturer’s study in rats in which 300 mg MPEP/kg body weight/day (mg/kg/day) was associated with a 15% decrease in body weight gain during pregnancy and dam mortality was observed at 1,000 mg/kg/day. A high dose of 500 mg/kg/day was selected because it was half the dose associated with dam mortality in the sponsoring manufacturer’s study, and likely high enough to ensure that the dams were challenged. The 125 and 250 mg/kg/day dose levels were similar to the 100 and 300 mg/kg/day dose levels in the sponsoring manufacturer’s study. Because of the availability of these summary data and the desire to generate timely information on potential hazard given human exposure, a dose range-finding study was not considered warranted. Owing to possible differences in rat sensitivity (strain or genetic drift) and to aid in identifying a no-observed-effect level for fetuses, one lower dose (62.5 mg/kg/day) was added. Oral gavage was selected as the route of administration given the short duration of dosing and that oral exposure is of most concern for humans. The route and corn oil vehicle would also allow comparison of the study findings with the sponsoring manufacturer’s summary data.
Prenatal Developmental Toxicity Study in Rats
On receipt (GD 1 or GD 2), time-mated rats were individually identified by tail marking and were randomized by GD 6 body weight stratification into five dose groups using the Instem™ Provantis® (version 8) electronic data collection system.
Groups of 25 time-mated female rats were administered 0 (vehicle control), 62.5, 125, 250, or 500 mg/kg/day (based on the most recent body weight), in corn oil by gavage from GD 6 to GD 20. Vehicle control animals received corn oil vehicle alone; the dosing volume was 2 mL/kg.
On GD 21, fetuses were removed from the uterus and live fetuses individually weighed. The uteri of animals that did not appear pregnant were examined for nidations (implantation sites) by staining with 10% ammonium sulfide.35,36 All fetuses were examined externally for alterations, including inspection of the oral cavity for cleft palate. Live fetuses were subsequently euthanized by intraperitoneal injection of sodium pentobarbital. Fetal sex was confirmed by inspection of gonads in situ. All fetuses were examined for soft tissue alterations under a stereomicroscope.37,38 The heads were removed from approximately half of the fetuses in each litter and fixed in Bouin’s solution and subsequently examined by free-hand sectioning.39 This technique precludes skeletal evaluations of the skull; therefore, remaining heads and all fetuses were eviscerated, fixed in ethanol, macerated in potassium hydroxide, stained with Alcian blue and Alizarin red, and examined for subsequent cartilage and osseous alterations.36,40 External, visceral, and skeletal fetal alterations were recorded as developmental variations or malformations.
On GD 18, blood was collected from dams in the 0, 62.5, and 250 mg/kg/day groups designated for biological sampling (n = 3 or 4 per dose group) approximately 2 hours postdose. Blood was collected via cardiac puncture into tubes containing tripotassium ethylenediaminetetraacetic acid (K3 EDTA). Following maternal blood collection, amniotic fluid was collected and pooled by litter. Fetuses were removed from amniotic sacs, euthanized by decapitation, collected, frozen, and pooled by litter. All blood samples from dams were collected within 2 hours of each other and kept on ice until processing. Blood samples were centrifuged (refrigerated), and the plasma was isolated and frozen at approximately −70°C. Plasma samples, amniotic fluid, and fetuses were shipped on dry ice to the analytical laboratory at RTI International (Research Triangle Park, NC). All samples were analyzed for MPEP concentration as described in Appendix D.
Dose Selection Rationale for the Dose Range-finding Study in Rabbits
Dose selection for the range-finding study was made on the basis of the sponsoring manufacturer’s study that used melted and cooled MPEP administered to rabbits by gavage at doses of 100, 300, and 1,000 mg/kg/day from GD 6 to GD 18.41 Moribundity and mortality were observed at 1,000 mg/kg/day. Other findings at this dose included soft stools, decreased feed consumption, decreased activity and bradypnea, and premature delivery/abortions. Similar findings, at lower incidences, were also observed in the 300 mg/kg/day dose group. Therefore, doses of 300, 400, and 500 mg/kg/day were selected to identify the highest tolerated dose rather than exploring an “optimal” dose-response relationship. The high dose of 500 mg/kg/day was one-half of the dose associated with unacceptable maternal toxicity; the two lower doses were selected to encompass possible rabbit stock differences.
Dose Range-finding Study in Rabbits
On receipt (GD 1 or GD 2), time-mated rabbits were individually identified by ear marking and were randomized by GD 6 body weight stratification into four dose groups using the Instem™ Provantis® (version 9) electronic data collection system.
Groups of eight time-mated female rabbits were administered 0 (vehicle control), 300, 400, or 500 mg/kg/day MPEP (based on the most recent body weight) in corn oil by gavage from GD 7 to GD 28. Vehicle control animals received corn oil vehicle alone; the dosing volume was 2 mL/kg.
On GD 29, fetuses were removed from the uterus, individually weighed (live fetuses only), and examined externally for alterations, including inspection of the oral cavity for cleft palate. Live fetuses were euthanized by intraperitoneal injection of a commercially available solution containing sodium pentobarbital. Fetuses were not retained after completion of the external examination.
Prenatal Developmental Toxicity Study in Rabbits
On receipt (GD 1 or GD 2), time-mated rabbits were individually identified by ear marking and were randomized by GD 6 body weight stratification into five dose groups using the Instem™ Provantis® (version 9) electronic data collection system.
Groups of 23 or 24 time-mated female rabbits were administered 0 (vehicle control), 62.5, 125, or 250 mg/kg/day MPEP (based on the most recent body weight) in corn oil by gavage from GD 7 to GD 28. Vehicle control animals received corn oil vehicle alone; the dosing volume was 2 mL/kg.
On GD 29, fetuses were removed from the uterus, and live fetuses were individually weighed. The uteri of animals that did not appear pregnant were examined for nidations (implantation sites) by staining with 10% ammonium sulfide.35,36 All fetuses were examined externally for alterations, including inspection of the oral cavity for cleft palate. Live fetuses were subsequently euthanized by intraperitoneal injection of sodium pentobarbital. Fetal sex was confirmed by inspection of gonads in situ. All fetuses were examined for soft tissue alterations under a stereomicroscope, including a soft tissue examination of the head.37,38 All fetuses were eviscerated, fixed in ethanol, macerated in potassium hydroxide, stained with Alcian blue and Alizarin red, and examined for subsequent cartilage and osseous alterations.36,40 External, visceral, and skeletal fetal alterations were recorded as developmental variations or malformations. Over-maceration during skeletal processing occurred in all groups: (1) vehicle control group (47 fetuses [F] from 5 litters [L]), limited to phalanges (22F, 3L), with fore/hind limbs (22F, 3L), and included portions of the axial skeleton (7F, 2L); (2) 62.5 mg/kg/day dose group (2F, 2L), limited to phalanges (1F), and included the fore/hind limbs (1F); (3) 125 mg/kg/day dose group (51F, 4L), limited to phalanges (13F, 2L), included fore/hind limbs (21F, 4L), and portions of the axial skeleton (3F, 1L); (4) 250 mg/kg/day dose group (10F, 1L), limited to phalanges (1F), and included the fore/hind limbs (9F, 1L). These structures were removed from the respective incidence calculations, and their removal was not considered to affect the study results.
On GD 27, blood was collected from does designated for biological sampling (n = 3 per dose group) at four time points (before dosing and at 4, 8, and 24 hours postdose). Collection at 24 hours occurred before dosing on GD 28. On GD 28, approximately 2 hours after dosing, blood was collected from the same does and their fetuses. For does, blood was collected from the central ear artery or lateral ear vein into tubes containing K3 EDTA. After their blood collection, does were euthanized and trunk blood was collected from each fetus. All samples were collected approximately 2 hours after the last dose and within 2 hours of each other and kept on ice until processing. Blood samples were centrifuged (refrigerated), and the plasma was isolated and frozen at approximately −70°C. Samples were shipped on dry ice to the analytical laboratory at RTI International (Research Triangle Park, NC). All samples were analyzed for MPEP concentration as described in Appendix D.
Statistical Methods
In the prenatal developmental toxicity study in rats and the dose range-finding and prenatal developmental toxicity studies in rabbits, statistical analyses were performed on data from pregnant females that survived until study termination and were examined on GD 21 (rats) or GD 29 (rabbits) and from live fetuses. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Descriptive Statistics
Maternal Parameters: Maternal body weights are reported as means. Terminal maternal body weights at GD 21 (for rats) or GD 29 (for rabbits) were adjusted for gravid uterine weight by subtracting the gravid uterine weight from the dam’s (or doe’s) body weight. Body weight gains were calculated over each 3-day interval and from GD 6 through GD 21 (or GD 7 through GD 29). Daily feed consumption was averaged over each 3-day interval and from GD 6 through GD 21 (or GD 7 through GD 29). These continuous variables, in addition to gravid uterine weights and other organ weights, are summarized with means and standard errors.
Placental and Fetal Parameters: Data on uterine contents are reported as means and standard errors of counts per dam per litter or doe per litter (corpora lutea, implants, resorptions, dead fetuses) and as total numbers of occurrences (resorptions, dead fetuses). Data from females that were not pregnant or that did not survive to study termination were not included. Postimplantation loss is calculated as a percentage of the number of implants per dam or per doe. Fetal findings are reported as means and standard errors of counts per litter (numbers of live fetuses, male fetuses, female fetuses), means and standard errors of litter means (fetal weight, male fetal weight, female fetal weight) and total numbers of occurrences (total number of live fetuses). In addition, several calculated variables are reported, including the percentage of live male and female fetuses per litter.
Incidences of morphological findings from the gross, external, visceral, skeletal, and head examinations of pathology of placentae and fetuses (where applicable) are presented as number and percentage of affected fetuses per exposure group and as number and percentage of affected litters per exposure group.
Analysis of Maternal Parameters and Uterine Contents
Maternal organ and body weight and fetal body weight data, which historically have approximately normal distributions, were analyzed with the parametric multiple comparison procedures of Dunnett and Williams.42 Nonnormally distributed variables, such as feed consumption and uterine content endpoints, were analyzed using the nonparametric multiple comparison methods of Shirley43 (as modified by Williams44) and Dunn.45 For all the continuous endpoints, the Jonckheere test46 was used to assess the significance of dose-related trends at p < 0.01 to determine whether a trend-sensitive test (Williams or Shirley test) was more appropriate than a test that does not assume a monotonic dose-related trend (the Dunnett or Dunn test). Before statistical analysis, extreme values identified by the outlier test of Dixon and Massey47 for small samples (n < 20) and Tukey’s outer fences method48 for large samples (n ≥ 20) were examined by NTP personnel, and implausible values were eliminated from the analysis.
Incidences of gross findings in the dams or does (binary endpoints) were analyzed using the Cochran-Armitage trend test49 and Fisher’s exact test.50
Analysis of Fetal Findings
The tendency of littermates to respond more similarly than animals in different litters has been referred to as the “litter effect”51 and reflects littermates’ similarities in genetics and early life environment. Failure to account for correlation within litters leads to underestimates of variance in statistical tests, resulting in higher probabilities of Type I errors (“false positives”). To accommodate litter effects in the fetal findings data, the Cochran-Armitage test was modified using the Rao-Scott approach.52 The Rao-Scott approach accounts for litter effects by estimating the ratio of the variance in the presence of litter effects to the variance in the absence of litter effects. This ratio is then used to adjust the sample size downward to yield the estimated variance in the presence of litter effects. The Rao-Scott approach was implemented in the Cochran-Armitage test as recommended by Fung et al.,53 formula ₸RS2.
Historical Control Data
The concurrent control group is the most valid comparison to the treated groups and is the only control group analyzed statistically in NTP developmental and reproductive toxicity studies. However, historical control data are often helpful in interpreting potential exposure-related effects, particularly for uncommon fetal findings that occur at a very low incidence. For meaningful comparisons, the conditions for studies in the historical control database must be generally similar. Factors that might affect the background incidences of fetal findings at a variety of sites are diet, strain/stock, route of exposure, study type, and/or laboratory that conducted the study. The NTP historical control database for teratology studies contains all fetal evaluations (e.g., teratology studies or modified one-generation studies) for each laboratory. In general, the historical control database for a given study includes studies using the same route of administration and study design. However, historical control data for rats in this NTP Developmental and Reproductive Toxicity Technical Report contain data from gavage studies conducted at Southern Research. The concurrent controls are included in the historical control data set. There are no NTP historical control data available for rabbits. NTP historical controls are available online at https://ntp.niehs.nih.gov/data/controls/index.html.
Quality Assurance Methods
The prenatal developmental toxicity study in rats and the dose range-finding and prenatal developmental toxicity studies in rabbits were conducted in compliance with U.S. Food and Drug Administration Good Laboratory Practice Regulations (21 CFR Part 58).54 Records from these studies were submitted to the NTP Archives. The prenatal developmental toxicity study in rats and the dose range-finding and prenatal developmental toxicity studies in rabbits were audited retrospectively by an independent quality assessment contractor. Separate audits covered completeness and accuracy of the final study data tables for the dose range-finding and prenatal developmental toxicity studies and a draft of this NTP Developmental and Reproductive Toxicity Technical Report. Audit procedures and findings are presented in the reports and are on file at NIEHS. The audit findings were reviewed and assessed by NTP staff, and all comments were resolved or otherwise addressed during the preparation of this report.
Results
Data Availability
The National Toxicology Program (NTP) evaluated all study data. Data relevant for evaluating toxicological findings are presented here. All study data are available in the NTP Chemical Effects in Biological Systems (CEBS) database: https://doi.org/10.22427/NTP-DATA-DART-07.55
Prenatal Developmental Toxicity Study in Rats
Maternal Findings
Viability and Clinical Observations
All rats survived until study termination with the exception of one rat removed early due to a dose administration error (Table 3). No clinical observations were attributed to 2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) administration (Appendix F). Red vaginal discharge was observed in one dam in the 125 mg MPEP/kg body weight/day (mg/kg/day) on gestation day (GD) 19 and at necropsy on GD 21; the same dam exhibited nidation sites after uterine staining. Given the singular incidence and no effect on mean postimplantation loss, this observation was considered an incidental response. One dam in the 500 mg/kg/day dose group delivered prior to scheduled necropsy on GD 21.
Body Weights and Feed Consumption
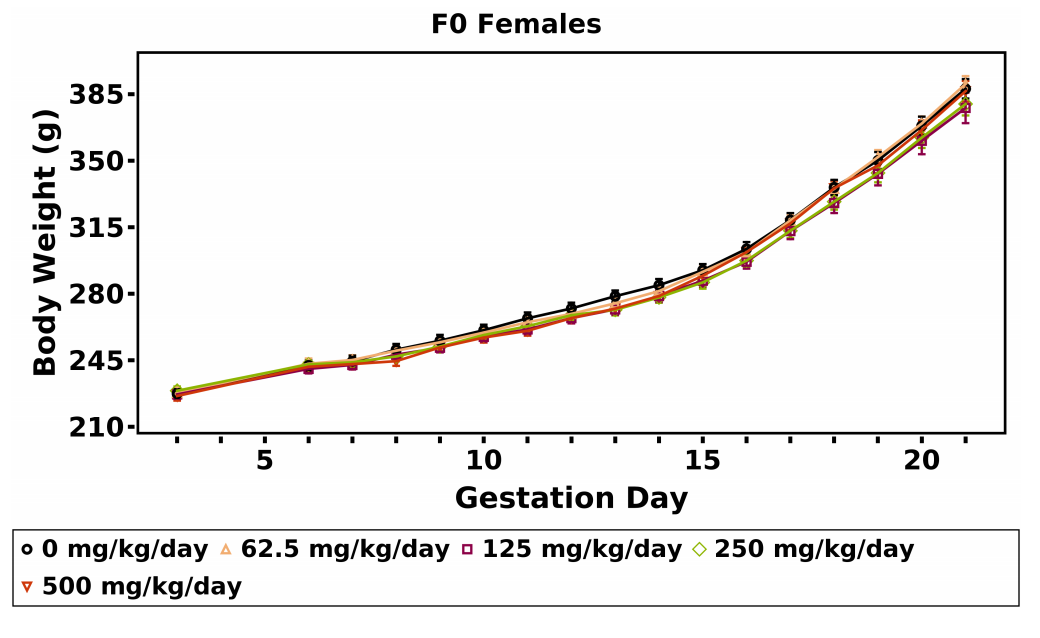
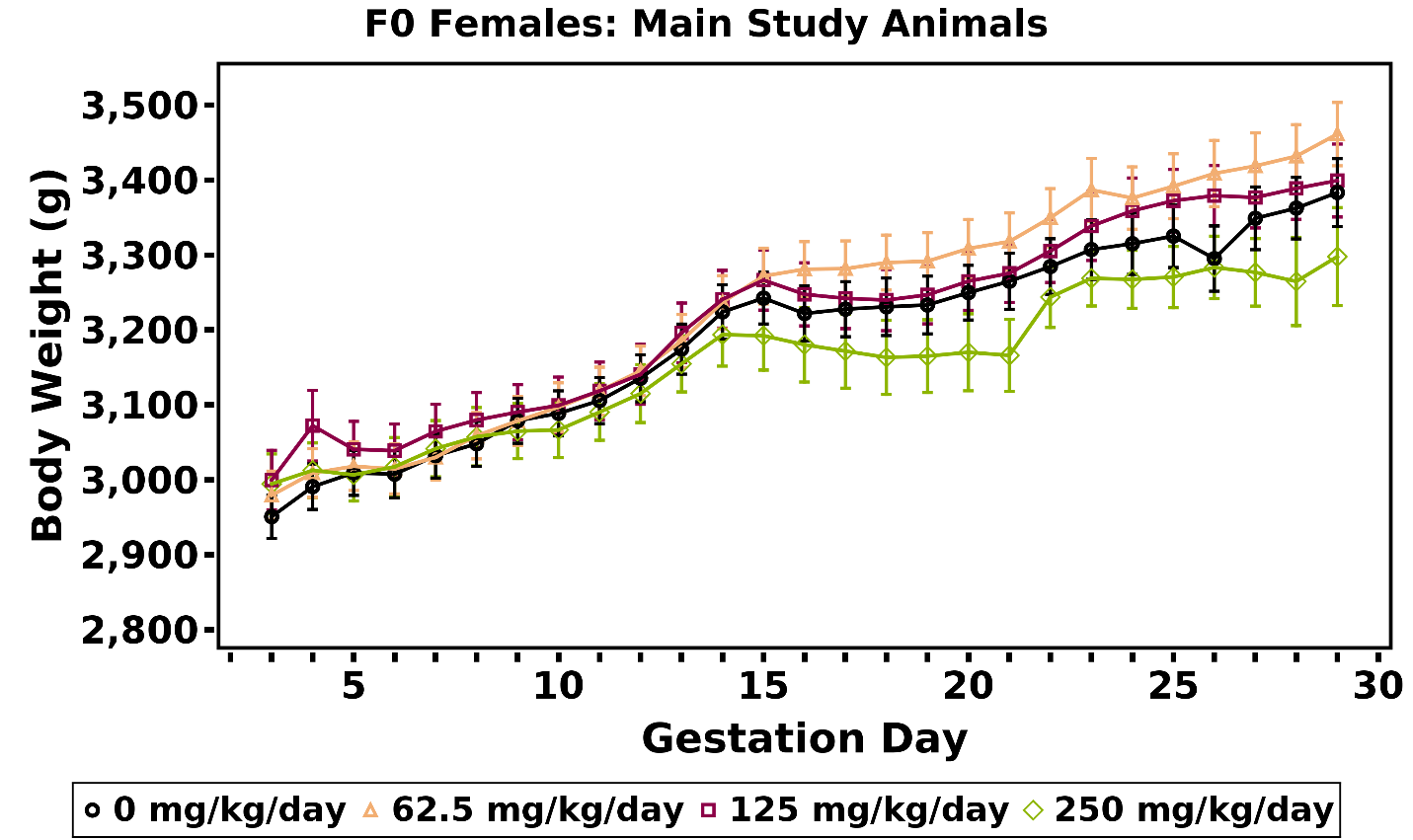
A significant decrease in mean body weight gain (22%–28%) relative to the vehicle control group was observed in the 250 and 500 mg/kg/day dose groups over the GD 6–9 interval. Subsequent mean body weights and body weight gains of the MPEP dose groups were similar to those of the vehicle control group (Table 4; Figure 4). No MPEP-related effect on GD 6–21 mean body weight or body weight gains adjusted for gravid uterine weight (at necropsy) was observed. Daily mean body weights and body weight gains of dams in each dose group are available in Appendix F.
A concomitant dose-related decrease (~11%) in maternal feed consumption was observed in the 500 mg/kg/day dose group over the GD 6–9 interval (Table 5). Feed consumption by the 500 mg/kg/day dose group was generally higher, relative to the vehicle control group, over subsequent intervals; however, feed consumption by this group over the GD 6–21 interval was similar to that by the vehicle control group.
Maternal and Litter Observations
No gross observations at necropsy were related to MPEP administration (Appendix F). No effects of MPEP administration were observed on the numbers of resorptions or live fetuses (Table 6).
Male and female fetal weights in the 500 mg/kg/day group were slightly lower (<4%) than those of the vehicle control groups (significant trend and pairwise for female weight). This observation was associated with a slightly higher live litter size (approximately one fetus). Gravid uterine weight was not affected by MPEP exposure (Table 6). These apparent minimal responses were not considered adverse and were likely spurious.
Fetal Findings
External
No external malformations or variations were attributed to MPEP exposure at 62.5, 125, 250, or 500 mg/kg/day (Appendix F). External findings in exposed rat fetuses were limited to a singular occurrence of subcutaneous hemorrhage in the 250 mg/kg/day group and singular occurrences of thread-like tail in the 125 and 250 mg/kg/day groups; those occurrences were considered unrelated to MPEP exposure.
Visceral
No visceral malformations were attributed to MPEP exposure (Table 7; Appendix F). Singular incidences of misshapen liver lobes, a malformation, were observed in two litters in the 500 mg/kg/day group, one left lateral lobe and one left medial lobe. All groups, including the vehicle control group, displayed a low incidence of the malformation misshapen aortic valve. The malformation hemorrhagic stomach was observed at a singular incidence in two litters in the 250 mg/kg/day exposure group. Other malformations included a singular incidence of retroesophageal aortic arch (250 mg/kg/day), absent left subclavian artery (250 mg/kg/day), absent right subclavian artery (vehicle control group), malpositioned testis (62.5 mg/kg/day), hydronephrosis (62.5 mg/kg/day), and misshapen renal capsule (500 mg/kg/day). Hemorrhagic testes (one in the vehicle control group, two and three—all from different litters—in the 62.5 and 250 mg/kg/day groups, respectively) were also observed.
An increased incidence of discolored liver lobe, a variation, in fetuses exposed to 125 mg/kg/day or greater (fetal incidence of 0.34% to 1.51%; litter incidence of 4.76% to 15%; Table 7) was observed. This finding was not observed in the vehicle control group or historical control groups and is likely the result of fetal liver metabolism of MPEP. All MPEP-exposed groups displayed an increase in the incidence of an additional fissure in the left lateral lobe of the liver; however, that finding had been observed in vehicle control groups. When all the common liver fissure variants were combined, no dose-response relationship was observed, and the fetal and litter incidences in the MPEP-exposed groups were similar to those in the vehicle control group. Although some litters had fetuses with both fissures and lobe discoloration, the incidence was too low to demonstrate any direct association (Appendix F).
Head
No visible head lesions in any of the exposure groups were observed (Appendix F).
Skeletal
MPEP exposure was not associated with increased incidences of any skeletal malformations or variations (Appendix F).
Internal Dose Assessment
On GD 18, dam plasma, amniotic fluid (pooled by litter), and fetuses from the 0, 62.5, and 250 mg/kg/day groups were analyzed for MPEP concentrations. MPEP concentrations in dam plasma increased less than proportionally with increasing dose. MPEP was measured in fetuses and was 18%–26% of that in dam plasma, suggesting low to moderate gestational transfer of MPEP in rats. MPEP was observed in dam plasma (3.5 ng/mL) and fetuses (~50 ng/mL) from vehicle control groups (Table 8). Although the concentration in dam plasma was similar to the background concentrations observed in control rat plasma matrices used in analytical method development, the fetal concentrations were approximately 10-fold higher than the concentrations in the corresponding control fetal homogenate matrix.
Dose Range-finding Study in Rabbits
Maternal Findings
Viability and Clinical Observations
Rabbits dosed with 500 mg/kg/day MPEP displayed body weight loss over the GD 7–9 interval, lower feed consumption, and concomitantly less feces, resulting in the dose group being removed from the study on GDs 10 and 11. The 400 mg/kg/day dose group also displayed body weight loss that was associated with generally lower feed consumption and less feces, resulting in four does being removed from the study on GDs 13, 14, 22, and 23. The rest of the 400 mg/kg/day dose group was removed from the study on GDs 24 and 25. Two does in the 300 mg/kg/day dose group displayed similar responses and were removed on GDs 12 and 23 (Table 9).
Body Weights and Feed Consumption
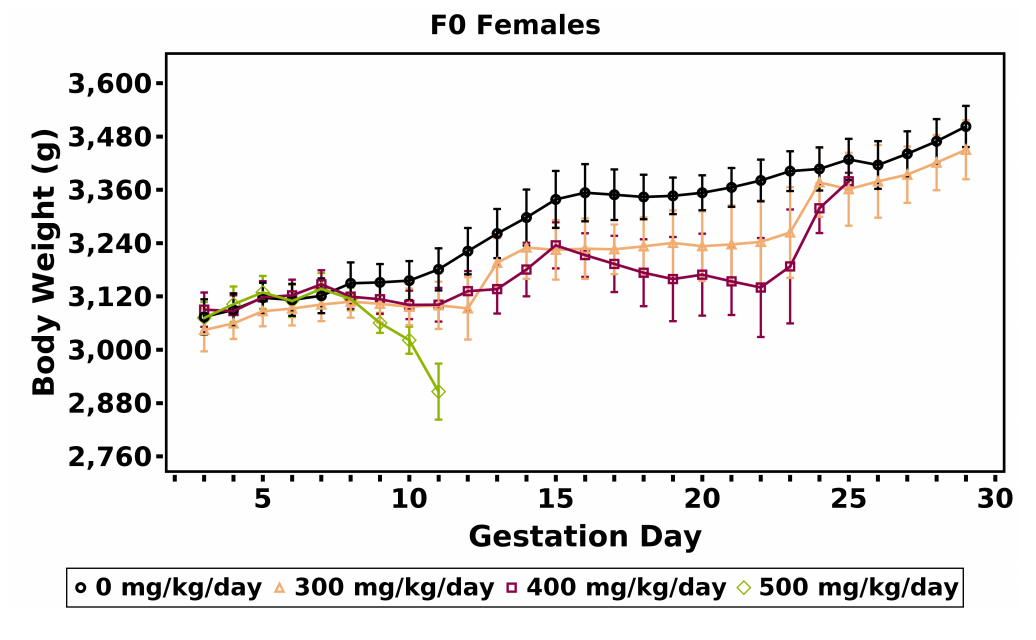
Mean body weight gain and mean feed consumption were slightly lower, relative to the vehicle control group, in the remaining 300 mg/kg/day does over the GD 7–9 and GD 9–12 intervals (Table 10, Table 11; Figure 5). However, those values included one doe that was removed on GD 12. The GD 7–29 body weight gains in the remainder of the 300 mg/kg/day dose group were similar to that of the vehicle control group (Table 10; Figure 5).
Maternal and Litter Observations
There were no MPEP-related gross observations in the 300 mg/kg/day group at necropsy.
No effects of 300 mg/kg/day MPEP on postimplantation loss or litter size were observed. Male and female fetal weights in the 300 mg/kg/day dose group were lower (12% and 9%, respectively) than those of the vehicle control group. These lower fetal weights were associated with a reduction in gravid uterine weight (8%) (Table 12).
Fetal Findings
There were no exposure-related external findings.
Dose Selection Rationale for the Prenatal Developmental Toxicity Study in Rabbits
In the rabbit dose range-finding study, maternal toxicity was clearly observed at doses ≥400 mg/kg/day, and similar findings, but at a lower incidence, were observed in the 300 mg/kg/day dose group. Therefore, 250 mg/kg was selected as the high dose for the prenatal developmental toxicity study, and half dose spacing was used to provide adequate spacing for evaluation of potential dose-response relationships and ideally to capture a no-observed-effect level. The doses selected for the prenatal developmental toxicity study were 62.5, 125, and 250 mg/kg/day.
Prenatal Developmental Toxicity Study in Rabbits
Maternal Findings
Viability and Clinical Observations
Three does in the 250 mg/kg/day dose group were removed from the study on GD 21 due to poor feed consumption with a concomitant decrease in feces and body weight. One doe in the 125 mg/kg/day dose group was removed from the study on GD 23 after displaying a similar response. Three does, one in the 125 mg/kg/day dose group and two in the 250 mg/kg/day dose group, delivered prior to necropsy; one doe in each of these groups began delivery just prior to scheduled necropsy. The other doe in the 250 mg/kg/day group gave birth on GD 28; this 1-day advancement was a single incidence and potentially the result of mistiming of insemination by the rabbit supplier rather than an MPEP-related effect. Three does, one in the 62.5 mg/kg/day dose group and two in the 250 mg/kg/day dose group, were not pregnant (Table 13).
Body Weights and Feed Consumption
Maternal body weight gains during gestation were generally lower in the 250 mg/kg/day dose group; however, mean body weight of this group remained within 3% of the vehicle control group (Table 14; Appendix F). Further, the 250 mg/kg/day groupexhibited a downward shift in the body weight curve (Figure 6). This apparent response was still present when the does that were euthanized prior to GD 29 were omitted. Body weight gain from GD 7 through GD 29 in the 250 mg/kg/day dose group was 20% lower than that of the vehicle control group, but the difference was not statistically significant (Table 14). The GD 29 body weights of the 250 mg/kg/day group were similar to those of the vehicle control group (Appendix F), as were the adjusted terminal body weights (Appendix F). In general, feed consumption (g/animal/day) by the 250 mg/kg/day dose group was decreased during the first week of dosing (Table 15). There were no biologically significant differences in mean body weight or feed consumption by the 62.5 and 125 mg/kg/day dose groups compared to the vehicle control group. Daily mean body weights of does in each dose group are available in Appendix F.
Maternal and Litter Observations
There were no notable maternal necropsy findings. The number of pregnant does available for examination was lower in the 250 mg/kg/day dose group due to early removals (16 versus 24 in the vehicle control group). The mean numbers of corpora lutea, implantation sites, and early and late resorptions were similar across groups (Table 16).
MPEP exposure did not affect the mean number of viable fetuses per litter, sex ratio, or significantly affect male or female fetal weight (Table 16).
Fetal Findings
External
MPEP exposure was not associated with increased incidences of any external malformations or variations. A singular occurrence of umbilical hernia was observed in the 62.5 mg/kg/day group (Appendix F).
Visceral
MPEP exposure was not associated with increased incidences of any visceral malformations or variations. Unilateral absent testes were observed in three fetuses from different litters in the 125 mg/kg/day group, but this finding was not observed in the 250 mg/kg/day group and has been observed once in recent historical control groups. Other findings occurred at a low incidence in all exposure groups (including the vehicle control group; e.g., testis, malpositioned; supernumerary thymus) or were found as a singular incidence (e.g., epididymis, left absent; hydronephrosis, right kidney), none of which suggest an exposure-response trend (Appendix F).
Head
The only head malformation observed in an MPEP-exposed group was a single incidence of hydrocephaly in one fetus in the 125 mg/kg/day group (Appendix F). This finding was incidental and not considered to be related to MPEP exposure.
Skeletal
Fetuses from the 125 and 250 mg/kg/day groups displayed an increase in the incidences of the malformations unilateral or bilateral costal cartilage seventh not fused to sternum (three fetuses from two litters and three fetuses from one litter, respectively) (Table 17). These findings (i.e., the absence of the underlying cartilaginous structures connecting the seventh rib to the sternum) were not observed in litters that were inadvertently over-macerated during skeletal processing. The incidence of 13th rib, unilateral or bilateral detached, was observed in all exposed groups and the vehicle control group. The 125 mg/kg/day group had the highest incidence (17 fetuses from 10 litters). Similarly, the malformation lumbar vertebra fused to ilium was observed at similar incidences in all exposure groups, including the vehicle control group (Appendix F).
Internal Dose Assessment
Maternal plasma on GDs 27 and 28 and fetal plasma on GD 28 were analyzed for MPEP concentrations (Table 18). Samples were collected at three timepoints that bracketed the time of anticipated maximum concentration to allow for the detection of MPEP in both maternal and fetal compartments. In general, MPEP concentrations in maternal plasma increased proportionally with the dose on both GD27 and GD 28. On GD 27, predose plasma had measurable concentrations of MPEP, demonstrating that MPEP was not completely cleared after 24 hours. The MPEP concentration was highest at 8 hours postdosing in all dosed groups suggesting the time to reach maximum concentration is ≥8 hours. On GD 28, MPEP concentrations in fetal plasma were similar to that of maternal plasma, demonstrating significant gestational transfer of MPEP. MPEP was observed in plasma from does (11–29 ng/mL) and fetuses (~10 ng/mL) from vehicle control groups; those concentrations were approximately two- to sixfold higher than the background concentrations observed in the respective control matrices used in analytical method development.
Zebrafish Assay Data
MPEP was included in an NTP high-throughput embryonic zebrafish assay to screen chemicals for their potential to cause developmental toxicity.56 This assay is particularly useful for evaluating adverse effects to the head or craniofacial region, which is a proposed target of MPEP-induced developmental toxicity in the literature. Following a 4-day exposure to MPEP (5–20 μM), microcephaly, heart edema, microphthalmia, yolk sac edema, trunk alterations (curved/curled), and yolk opacity were quantified in larvae (Appendix E). Mortality was observed in all embryos at concentrations ≥50 μM. The calculated median effective concentration (EC50) for MPEP was 5.4 μM (1.7 μg/mL). These results align with findings in other zebrafish embryo studies by Padilla et al.57 and Troung et al.58 The concentrations at which MPEP was associated with adverse morphological effects, including craniofacial deficits, was similar across studies despite different experimental approaches: median activity concentration (AC50) of 26 μM by Padilla and colleagues versus EC50 of 5.2 μM by Troung and colleagues.57,58 However, adverse fetal findings were not observed in NTP rat and rabbit studies at similar internal dose concentrations (e.g., rat fetus = plasma concentration of 1,418 ng/mL in the 250 mg/kg/day MPEP group; rabbit fetus = plasma concentration of 255 ng/mL in the 250 mg/kg/day MPEP group). Comparing the results among zebrafish, rats, and rabbits suggests potential species differences between lower vertebrate models, such as teleost fish species, and more established vertebrate models used to assess human-relevant outcomes. In addition, many of the morphological alterations, such as microcephaly, were observed in fish exposed to a variety of other environmental chemicals and pharmaceuticals tested by NTP (e.g., 47 of 88 chemicals evaluated caused at least one morphological alteration).59 These results could suggest that microcephaly may be a common chemical-mediated response in embryonic zebrafish screening assays and not entirely MPEP specific.
Discussion
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) is an insecticide that acts as a juvenile insect hormone analog and growth regulator, preventing insect larvae from developing into adults and rendering them unable to reproduce. MPEP can be formulated into a variety of dispersant products for both home and agricultural use.2,3 Adding MPEP to potable water in cisterns/barrels was approved by the World Health Organization in 2008 to control mosquito populations in Zika virus-endemic areas.4 Although prenatal toxicity studies were conducted using experimental animals by the insecticide’s sponsoring manufacturer for marketing approval, and these were considered acceptable by governmental health authorities and showed no apparent hazard,4,7 the public has expressed concern that human exposure to MPEP during pregnancy could contribute to the cranial malformations observed in babies born to women infected with the Zika virus. Prenatal toxicology study results that might inform public health decisions were not available in the public domain. Given this knowledge gap, the National Toxicology Program (NTP) initiated a series of studies to inform potential human hazard.
NTP conducted prenatal developmental toxicity studies with MPEP in two mammalian species, Sprague Dawley (Hsd:Sprague Dawley® SD®) rats and New Zealand White (Hra:NZW SPF) rabbits, to investigate the possibility that MPEP exposure might induce skeletal malformations. The potential for MPEP to induce external and visceral variations and malformations also was assessed. In parallel, plasma MPEP concentrations in pregnant rats and rabbits and fetuses of both species were determined.
Dose selection for the rat prenatal developmental toxicity study was partially informed by the U.S. Environmental Protection Agency’s summary of the manufacturer’s submission data, which indicated effects on maternal body weight at 300 mg MPEP/kg body weight/day (mg/kg/day) and mortality at 1,000 mg/kg/day MPEP. The current high dose of 500 mg/kg/day was half the dose associated with mortality, and the current 250 mg/kg/day dose was comparable to the dose that reduced maternal body weight. MPEP was well tolerated by the dams, which allowed for the complete evaluation of embryo-fetal development. The high dose of 500 mg/kg/day was associated with a transient decrease in maternal body weight gain and feed consumption after dose initiation, demonstrating some minimal maternal toxicity. Higher dose levels would likely have induced greater effects on body weight that could result in nonspecific fetal toxicity. Exposure to MPEP did not affect any pregnancy or litter parameters. Fetal weight trended lower with increasing dose; however, the effect in the 500 mg/kg/day group was minimal (<4%) and was associated with a slight increase in litter size. The trend in decreasing fetal weights was not associated with an increase in skeletal variants, a relational response that is often observed, indicating that this decrease in weight was likely spurious.
Fetal rat visceral findings included minimal higher incidences of liver variations, including additional fissures and discoloration. Fissures were observed in the left lateral, left medial, and right medial lobes of the liver, and, when combining all locations, no apparent effect of MPEP exposure on the incidence was observed. The combined incidence was similar to what had been observed in control groups. These data indicate that the apparent higher incidence of liver lobe fissures in the 500 mg/kg/day group was likely incidental and not attributed to MPEP exposure.
Liver discoloration, a variation, was observed in the left medial and left lateral lobes of several fetuses from different litters in the 250 and 500 mg/kg/day groups; this variation was also observed in one fetus in the 125 mg/kg/day group. Discoloration was not coincident with the presence of fissures. Liver lobe discoloration could be an incidental finding but may be an indirect effect of MPEP on fetal liver metabolism, or organ insult. Unlike the increased incidences of cervical vertebra findings that were reported in the manufacturer’s study,41 the incidences of cervical vertebrae malformations in the current study were lower in the MPEP-exposed groups than in the control group.
Dose range-finding and prenatal developmental toxicity studies were performed in the rabbit to determine whether MPEP induced malformations or variations in a second mammalian species. In the dose range-finding study, overt maternal toxicity was observed in the 400 and 500 mg/kg/day dose groups, resulting in those groups being removed from the study. A lower prevalence of moribundity/morbidity was observed in the 300 mg/kg/day dose group. Uterine and fetal weights were slightly lower in the 300 mg/kg/day dose group relative to the vehicle control group, but the minimal response could have been secondary to maternal toxicity. No external or placental observations were attributed to MPEP exposure. A high dose of 250 mg/kg/day was therefore selected for the prenatal developmental toxicity study to ensure sufficient challenge to the doe to increase identification of fetal alteration if present.
The dose of 250 mg/kg/day was generally well tolerated by most does, but a limited number in this group exhibited unacceptably lower body weight and feed consumption, resulting in three animals being removed from the study. Two does in the 250 mg/kg/day dose group delivered prior to necropsy. The five early removals and two nonpregnant does collectively resulted in 16 litters being available for examination. Litter size, postimplantation loss, and fetal weight were not affected by MPEP exposure. The findings indicate that the 250 mg/kg/day dose resulted in some limited maternal toxicity, thus the does and fetuses received the highest dose possible without affecting apical indictors of nonspecific, maternally induced, fetal toxicity.
Fetuses exposed to 125 and 250 mg/kg/day MPEP displayed a higher incidence in costal seventh cartilage not fused to sternum (malformation). The absence of this cartilaginous structure in the rabbit could be a developmental delay resulting from localized changes in chondrocyte proliferation.60-62 Feed consumption by, and mean body weight gains of, these does were similar to does for which fetuses did not display the malformation, suggesting that this finding is not the result of maternal toxicity. This structural defect is recognized by the International Federation of Teratology Societies.32,63 This finding is not specifically listed in the publicly available New Zealand White rabbit historical control data from Charles River’s commercial contract laboratories; however, the malformation “costal cartilage anomaly” has been observed at a very low incidence in control animals (17/15,511 [fetuses]; 13/1,759 [litters]).64 These marginal effects, which may or may not be related to MPEP exposure, are considered equivocal evidence of developmental toxicity.
MPEP concentrations were measured in both maternal and fetal samples in rats and rabbits following exposure to MPEP. In rats, maternal MPEP concentrations in plasma were 91- and 26-fold higher and fetal concentrations were 13- and 6-fold higher than those in rabbits for the 62.5 and 250 mg/kg/day groups, respectively. In rats, fetal plasma concentrations were 18%–26% of the concentrations in dams suggesting moderate gestational transfer of MPEP. Unlike rats, fetal concentrations in rabbits were similar to maternal concentrations demonstrating considerable transfer of MPEP from does to fetuses. The increase in MPEP concentration in fetuses was minimal between 125 and 250 mg/kg groups and may be a plausible explanation for similar fetal malformations observed between the groups. MPEP was observed in matrices from vehicle control groups. However, the concentrations of MPEP in the rat feed used in these studies were confirmed to be below the limit of quantitation of the feed analysis method of 0.01 mg/kg feed; MPEP concentrations were not measured in the rabbit feed. Therefore, potential low-level exposure of study animals to MPEP via feed cannot be ascertained. In rats, the MPEP concentration in control fetuses was much higher than that in respective dams, whereas in the dosed groups the reverse was observed, suggesting that the concentration of MPEP observed in control groups was mostly introduced poststudy during sample preparation and analysis. In rabbits, the MPEP concentration in fetuses and does was similar across the control and dosed groups, suggesting potential low-level exposure to MPEP or related compounds. Current EPA individual tolerances for MPEP present in non-grass animal feed range from 0.7 to 2.0 ppm; therefore the presence of MPEP in control animal samples is not unexpected.
Microcephaly was observed in the NTP zebrafish model; however, this finding is possibly coincidental with yolk sac anomalies and should not be considered informative on potential human hazard. The log KOW of MPEP is >5, suggesting that it can bioaccumulate in the lipids in the egg yolk, resulting in much higher exposures of zebrafish embryos than would be expected in placental mammals.
Collectively, the rat and rabbit fetal examination data do not suggest that MPEP is teratogenic in mammals, although it does induce morphological alterations in zebrafish embryos. Thus, the results of these rat and rabbit studies do not support the hypothesis that MPEP is the direct cause of the microcephaly observed in Zika virus-endemic areas in which MPEP is used as an insecticide to control mosquito populations. The difference between the zebrafish findings and the findings in rats and rabbits suggests potential species differences between lower vertebrate models and more established vertebrate models used to assess human-relevant outcomes. The results in zebrafish could suggest that microcephaly may be a common chemical-mediated response in embryonic zebrafish screening assays and not entirely MPEP specific.
References
1. National Center for Biotechnology Information (NCBI). PubChem Compound Summary for Pyriproxyfen. Bethesda, MD: National Center for Biotechnology Information; 2020. https://pubchem.ncbi.nlm.nih.gov/compound/91753
2. National Center for Biotechnology Information (NCBI). PubChem Pyriproxyfen: 8.3 USDA Pesticide Data Program. Bethesda, MD: National Center for Biotechnology Information; 2020. https://pubchem.ncbi.nlm.nih.gov/compound/Pyriproxyfen#section=USDA-Pesticide-Data-Program
3. U.S. Environmental Protection Agency (USEPA). Letter to Valent USA Corporation regarding label amendments. Washington, DC: U.S. Environmental Protection Agency, Office of Prevention Pesticides and Toxic Substances; 2012. https://www3.epa.gov/pesticides/chem_search/ppls/059639-00160-20120717.pdf
4. World Health Organization (WHO). Pyriproxyfen in drinking-water: Use for vector control in drinking-water sources and containers. 2008. https://www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/pyriproxyfen-background.pdf?ua=1
5. Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016; 29(3):487-524. DOI: 10.1128/CMR.00072-15 PubMed: 27029595
6. Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017; 390(10107):2099-2109. DOI: 10.1016/S0140-6736(17)31450-2 PubMed: 28647173
7. Code of Federal Regulations (CFR). 2002; 40(Part 180).
8. Red Universitaria de Ambiente y Salud (REDUAS). Report from physicians in the crop-sprayed town regarding Dengue-Zika, microcephaly, and massive spraying with chemical poisons. 2016; https://reduas.com.ar/report-from-physicians-in-the-crop-sprayed-town-regarding-dengue-zika-microcephaly-and-massive-spraying-with-chemical-poisons/
9. Reis V. Nota técnica sobre microcefalia e doenças vetoriais relacionadas ao Aedes aegypti: os perigos das abordagens com larvicidas e nebulizações químicas – fumacê. 2016. https://www.abrasco.org.br/site/outras-noticias/institucional/nota-tecnica-sobre-microcefalia-e-doencas-vetoriais-relacionadas-ao-aedes-aegypti-os-perigos-das-abordagens-com-larvicidas-e-nebulizacoes-quimicas-fumace/15929/
10. Parens R, Nijhout HF, Morales A, Xavier Costa F, Bar-Yam Y. A possible link between pyriproxyfen and microcephaly. PLoS currents. 2017; 9:ecurrents.outbreaks.5afb0bfb8cf31d39a34baba37b19b34edbac.
11. World Health Organization (WHO). Guidelines for drinking-water quality. Third edition incorporating the first and second addenda. Volume 1. Recommendations. Geneva; 2008.
12. Matsunaga H, Yoshino H, Isobe N, Kaneko H, Nakatsuka I, Yamada H. Metabolism of pyriproxyfen in Rats. 1. Absorption, disposition, excretion, and biotransformation studies with [phenoxyphenyl-14C]pyriproxyfen. J Agric Food Chem. 1995; 43(1):235-240. DOI: 10.1021/jf00049a042
13. Yoshino H, Kaneko H, Nakatsuka I, Yamada H. Metabolism of pyriproxyfen. 3. In vitro metabolism in rats and mice. J Agric Food Chem. 1996; 44(6):1578-1581. DOI: 10.1021/jf950510q
14. U.S. Environmental Protection Agency (USEPA). Data evaluation record: Sumilarv. Study type: Developmental toxicity study in rats (segments II and III). Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. 68D10075. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-018.pdf
15. U.S. Environmental Protection Agency (USEPA). Data evaluation record: Sumilarv technical. Study type: Reproductive toxicity. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. 68D10075. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-021.pdf
16. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Teratogenicity-developmental toxicity, rabbit. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-015.pdf
17. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Acute oral toxicity in rats. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. D179381. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-009.pdf
18. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Acute dermal toxicity in rats. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. D179381. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-014.pdf
19. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Acute inhalation toxicity in rats. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. D179381. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-013.pdf
20. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Eye irritation study in rabbits. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. D179381. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-011.pdf
21. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Skin irritation study in rabbits. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. D179381. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-012.pdf
22. Suzuki T. Sumilarv - Skin sensitization test with S-31183 in Guinea pigs (Original report written in Japanese, translated by Takachi Suzuki, May 14, 1987). Japan: Ministry of Agriculture, Forestry and Fisheries; 1987. NNT-70-0022. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-010.pdf
23. Code of Federal Regulations (CFR). Pyriproxyfen; Pesticide Tolerance. 1999; 40(Part 180).
24. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Oncogenicity study, mouse. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. D176679. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-020.pdf
25. U.S. Environmental Protection Agency (USEPA). Data evaluation report: Sumilarv (S-31183). Study type: Rat chronic feeding and oncogenicity-(83-5). Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-024.pdf
26. U.S. Environmental Protection Agency (USEPA). Data evaluation report: S-31183. Study type: Subchronic 90-day oral toxicity. U.S. Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-008.pdf
27. U.S. Environmental Protection Agency (USEPA). Data evaluation report: S-31183. Study type: 90-day feeding studies-non-rodent. U.S. Environmnetal Protection Agency, Office of Pesticide Programs, Health Effects Division; 1993. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-016.pdf
28. Chernoff N, Setzer RW, Miller DB, Rosen MB, Rogers JM. Effects of chemically induced maternal toxicity on prenatal development in the rat. Teratology. 1990; 42(6):651-658. DOI: 10.1002/tera.1420420610 PubMed: 2087686
29. U.S. Environmental Protection Agency (USEPA). Guidelines for developmental toxicity risk assessment. Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum; 1991. EPA Document No. EPA/600/FR-91/001.
30. Tyl RW. Commentary on the role of maternal toxicity on developmental toxicity. Birth Defects Res B Dev Reprod Toxicol. 2012; 95(3):262-266. DOI: 10.1002/bdrb.21015 PubMed: 22706915
31. Organisation for Economic Cooperation and Development (OECD). OECD guideline for the testing of chemicals, test no. 44, prenatal developmental toxicity study. Paris, France: Organisation for Economic Co-operation and Development; 2001.
32. Makris SL, Solomon HM, Clark R, Shiota K, Barbellion S, Buschmann J, Ema M, Fujiwara M, Grote K, Hazelden KP. Terminology of developmental abnormalities in common laboratory mammals (version 2). Congenit Anom (Kyoto). 2009; 49(3):123-246. DOI: 10.1111/j.1741-4520.2009.00239.x PubMed: 20002907
33. Suckow MA, Weisbroth S, Franklin C. The Laboratory Rat. Cambridge, MA: Academic Press; 2005.
34. Hayes AW, Kruger CL. Hayes’ Principles and Methods of Toxicology. Boca Raton, FL: CRC Press; 2014. DOI: 10.1201/b17359
35. Salewski E. Färbemethode Zum Makroskopischen Nachweis von Implantationsstellen am Uterus der Ratte. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964; 247(4):367-367. DOI: 10.1007/BF02308461
36. Tyl RW, Marr MC. Developmental toxicity testing-Metholodogy In: Hood RD, editor. Developmental and Reproductive Toxicology A Practical Approach. 2006.
37. Staples RE. Detection of visceral alterations in mammalian fetuses. Teratology. 1974; 9(3):259-260. PubMed: 4832056
38. Stuckhardt JL, Poppe SM. Fresh visceral examination of rat and rabbit fetuses used in teratogenicity testing. Teratog Carcinog Mutagen. 1984; 4(2):181-188. DOI: 10.1002/tcm.1770040203 PubMed: 6145223
39. Thompson RF. Chapter 4, Basic neuroanatomy. Foundations of Physiological Psychology. New York, NY: Harper and Row Publishers; 1967. p. 79-82.
40. Marr MC, Price CJ, Myers CB, Morrissey RE. Developmental stages of the CD® (Sprague-Dawley) rat skeleton after maternal exposure to ethylene glycol. Teratology. 1992; 46(2):169-181. DOI: 10.1002/tera.1420460210 PubMed: 1440420
41. Hirohashi A. Study of S-31183 by oral administration during the period of fetal organogenesis in rabbits. 1989; https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/129032/129032-015.pdf
42. Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955; 50(272):1096-1121. DOI: 10.1080/01621459.1955.10501294
43. Shirley E. A non-parametric equivalent of William’s test for contrasting increasing dose levels of treatment. Biometrics. 1977; 33:386-389. DOI: 10.2307/2529789 PubMed: 884197
44. Williams DA. A note on Shirley’s nonparametric test for comparing several dose levels with a zero-dose control. Biometrics. 1986; 42:183-186. DOI: 10.2307/2531254 PubMed: 3719054
45. Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964; 6(3):241-252. DOI: 10.1080/00401706.1964.10490181
46. Jonckheere A. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954; 41:133-145. DOI: 10.1093/biomet/41.1-2.133
47. Dixon WJ, Massey FJJr. Introduction to Statistical Analysis. New York, NY: McGraw-Hill Book Company, Inc; 1957. DOI: 10.2307/2332898
48. Tukey J. Easy summaries – numerical and graphical. Exploratory Data Analysis. Reading, MA: Addison-Wesley; 1977. p. 43-44.
49. Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955; 11(3):375-386. DOI: 10.2307/3001775
50. Gart JJ, Chu KC, Tarone RE. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. J Natl Cancer Inst. 1979; 62(4):957-974. PubMed: 285297
51. Kupper LL, Portier C, Hogan MD, Yamamoto E. The impact of litter effects on dose-response modeling in teratology. Biometrics. 1986; 42(1):85-98. DOI: 10.2307/2531245 PubMed: 3719065
52. Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics. 1992; 48(2):577-585. DOI: 10.2307/2532311 PubMed: 1637980
53. Fung KY, Krewski D, Rao JN, Scott AJ. Tests for trend in developmental toxicity experiments with correlated binary data. Risk Anal. 1994; 14(4):639-648. DOI: 10.1111/j.1539-6924.1994.tb00277.x PubMed: 7972964
54. Code of Federal Regulations (CFR). 21(Part 58).
55. National Toxicology Program (NTP). DART-07: Developmental and Reproductive Toxicity: Growth and clinical finding tables (I), pathology tables (PA), developmental and reproductive tables (R) from NTP developmental and reproductive toxicity studies. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 2020. DOI: 10.22427/NTP-DATA-DART-07
56. National Toxicology Program (NTP). Data release: Developmental neurotoxicity data integration and visualization enabling resource (DNT-DIVER). Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. DOI: 10.22427/NTP-DATA-002-00062-0001-0000-1
57. Padilla S, Corum D, Padnos B, Hunter DL, Beam A, Houck KA, Sipes N, Kleinstreuer N, Knudsen T, Dix DJ, et al. Zebrafish developmental screening of the ToxCast™ Phase I chemical library. Reprod Toxicol. 2012; 33(2):174-187. DOI: 10.1016/j.reprotox.2011.10.018 PubMed: 22182468
58. Truong L, Gonnerman G, Simonich MT, Tanguay RL. Assessment of the developmental and neurotoxicity of the mosquito control larvicide, pyriproxyfen, using embryonic zebrafish. Environ Pollut. 2016; 218:1089-1093. DOI: 10.1016/j.envpol.2016.08.061 PubMed: 27593350
59. National Toxicology Program (NTP). Screen for developmental toxicity in zebrafish-study 1. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. DOI: 10.22427/NTP-DATA-002-00077-0001-0000-7
60. Enomoto M, Pan H, Suzuki F, Takigawa M. Physiological role of vitamin A in growth cartilage cells: Low concentrations of retinoic acid strongly promote the proliferation of rabbit costal growth cartilage cells in culture. J Biochem. 1990; 107(5):743-748. DOI: 10.1093/oxfordjournals.jbchem.a123119 PubMed: 2398039
61. Enomoto M, Kinoshita A, Pan HO, Suzuki F, Yamamoto I, Takigawa M. Demonstration of receptors for parathyroid hormone on cultured rabbit costal chondrocytes. Biochem Biophys Res Commun. 1989; 162(3):1222-1229. DOI: 10.1016/0006-291X(89)90804-8 PubMed: 2548491
62. Takigawa M, Enomoto M, Shirai E, Nishii Y, Suzuki F. Differential effects of 1 alpha,25-dihydroxycholecalciferol and 24R,25-dihydroxycholecalciferol on the proliferation and the differentiated phenotype of rabbit costal chondrocytes in culture. Endocrinology. 1988; 122(3):831-839. DOI: 10.1210/endo-122-3-831 PubMed: 3257732
63. The DevTox Project. DevTox.Nomenclature. Berlin, Germany: German Federal Institute for Risk Assessment (BfR); 2012. https://www.devtox.org/nomenclature/ml_organ.php?lan=en
64. Charles River. Historical control data in New Zealand white rabbits. 2017; https://www.criver.com/sites/default/files/noindex/historical-control-data/embryo-fetal-hcd-ashland-rabbits.pdf
65. LabDiet. Certified high fiber rabbit diet. 2012; http://www.labsupplytx.com/wp-content/uploads/2013/07/5325-Certified-High-Fiber-Rabbit-Diet.pdf
66. Nishimura Y, Inoue A, Sasagawa S, Koiwa J, Kawaguchi K, Kawase R, Maruyama T, Kim S, Tanaka T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit Anom (Kyoto). 2016; 56(1):18-27. DOI: 10.1111/cga.12142 PubMed: 26537640
67. Lieschke GJ, Currie PD. Animal models of human disease: Zebrafish swim into view. Nat Rev Genet. 2007; 8(5):353-367. DOI: 10.1038/nrg2091 PubMed: 17440532
68. Dzieciolowska S, Larroque AL, Kranjec EA, Drapeau P, Samarut E. The larvicide pyriproxyfen blamed during the Zika virus outbreak does not cause microcephaly in zebrafish embryos. Sci Rep. 2017; 7:40067. DOI: 10.1038/srep40067 PubMed: 28051181
69. Quevedo C, Behl M, Ryan K, Paules RS, Alday A, Muriana A, Alzualde A. Detection and prioritization of developmentally neurotoxic and/or neurotoxic compounds using zebrafish. Toxicol Sci. 2019; 168(1):225-240. DOI: 10.1093/toxsci/kfy291 PubMed: 30521027
Conclusions
Under the conditions of the rat prenatal developmental toxicity study, there was no evidence of developmental toxicity of 2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) in Sprague Dawley (Hsd:Sprague Dawley® SD®) rats administered 62.5, 125, 250, or 500 mg/kg/day based on the absence of effects on reproductive parameters, fetal weight, or increased incidence of fetal malformations or variations. The highest dose administered was 500 mg/kg/day, which did not result in overt maternal toxicity.
Under the conditions of the rabbit prenatal developmental toxicity study, there was equivocal evidence of developmental toxicity of MPEP in New Zealand White (Hra:NZW SPF) rabbits based on the occurrence of the malformation “seventh costal cartilage not fused to sternum” in dosed groups. This finding was observed at 250 mg/kg/day, a dose that induced some maternal toxicity.
Appendices
Appendix A. Chemical Characterization and Dose Formulation Studies
A.1.
Procurement and Characterization
A.1.1.
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) was obtained from AK Scientific, Inc. (Union City, CA) in a single lot (JL44164). Identity, purity, and stability analyses were conducted by the analytical chemistry lab at RTI International (Research Triangle Park, NC). Reports on analyses performed in support of the MPEP studies are on file at the National Institute of Environmental Health Sciences.
MPEP was received as a white powder. The melting point of lot JL44164 was 46.6°C–47.9°C, which is consistent with literature values (45°C–47°C). Elemental analysis was consistent with the theoretical values of MPEP. Lot JL44164 was identified using infrared (IR) spectroscopy, 1H nuclear magnetic resonance (NMR) spectroscopy, and 13C NMR spectroscopy. Additionally, three types of 2-dimensional NMR were used to verify identity: proton-proton correlation spectroscopy (COSY), proton-carbon correlation spectroscopy (HSQC), and long-range proton-carbon correlation spectroscopy (HMBC). Gas chromatography (GC) with mass spectrometry detection (MS) also confirmed the identity of lot JL44164 (Table A-1, System A).
The IR spectrum was consistent with a reference spectrum of MPEP from the National Institute of Advanced Industrial Science and Technology (AIST) Spectral Database (No. 53368) (Figure A-1). Both 1H and 13C NMR spectra were in good agreement with library references from the AIST Spectral Database (No. HR2014-02914NS and CR2014-02914NS, respectively), as well as predicted spectra from Advanced Chemistry Development (ACD) spectral prediction program (Version 10.02, Toronto, Ontario, Canada) (Figure A-2). The high-resolution NMR techniques (COSY, HSQC, and HMBC) matched the ACD-predicted spectra and indicated impurity of <5%. The GC/MS spectra correlated well to the structure of MPEP.
The moisture content of lot JL44164 was determined by Karl Fisher titration. The purity of lot JL44164 was evaluated using GC with flame ionization detection (FID) and ultra-performance liquid chromatography (UPLC) with a photodiode array detector (PDA, 210 nm) (Table A-1, Systems B and C, respectively). Karl Fisher titration yielded water content of 0.03%. The GC/FID analysis determined the purity to be 99.6%; one impurity peak with 0.1% and seven peaks <0.1% of the total response were identified. The UPLC/PDA analysis determined purity to be 97.7% with two impurity peaks with 1.6% and 0.3% and 15 additional peaks <0.1%. The overall purity of lot JL44164 was estimated to be >97.7%.
Accelerated stability studies were conducted on samples of MPEP stored at 60°C, 22°C, 5°C, and −20°C using the UPLC/PDA described above. Stability was confirmed for at least 2 weeks when stored in sealed amber glass bottles sealed with Teflon-lined caps at 25°C, 5°C, and −20°C. Lot JL44164 was received in a 15 kg drum. Lot JL44164 was homogenized and transferred into 80-ounce bottles with Teflon sealed caps and stored at room temperature. Lot JL44164 was periodically analyzed (Table A-1, System D) to ensure no degradation relative to a frozen reference sample.
A.1.2.
Corn Oil
Corn oil was obtained from Welch, Holme & Clark Co. Inc (Newark, NJ) in a single lot (0120-0576) and used as a vehicle in the dose range-finding and prenatal developmental toxicity studies. A solubility and suspendability study of MPEP in corn oil determined that the test article was suspendable at up to 250 mg/mL and soluble at up to 136 mg/mL. Lot 0120-0576 contained peroxide levels that were less than the rejection level of 3 milliequivalents (meQ)/kg corn oil.
A.2.
Preparation and Analysis of Dose Formulations
Dose formulations of MPEP were prepared in corn oil following the protocols outlined in Table A-2. The rat and rabbit prenatal developmental toxicity studies used dose formulations of 31.25, 62.5, 125, and 250 mg/mL (rat only). Dose formulations of 150, 200, and 250 mg/mL were used in the rabbit dose range-finding study. Prior to study start, a homogeneity study at 250 mg/mL and a stability study at 1 mg/mL dose formulations were conducted using GC/FID (Table A-1, System D). Homogeneity and stability were confirmed for 42 days at room temperature (~25°C) when stored in clear glass bottles with Teflon-lined caps. To simulate conditions in the animal room, the 1 mg/mL formulation was stored in glass bottles at room temperature exposed to air and light for 3 hours. No loss in MPEP was found under these conditions.
Analysis of preadministration and postadministration dose formulations was conducted using GC/FID within 2 days of preparation and at the conclusion of the studies (Table A-3, Table A-4, Table A-5). Postadministration samples were collected from the remainder of the formulations in the day’s bottles. All dose formulation samples in the rat and rabbit prenatal developmental toxicity studies were within 10% of the target concentrations except for the 250 mg/mL postadministration sample in the rabbit dose range-finding study which was 21.3% above the target concentration.
Table A-1. Chromatography Systems Used in the Prenatal Developmental Toxicity Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| Chromatography | Detection System | Column | Mobile Phase |
|---|---|---|---|
| System A | |||
| Gas chromatography | Mass spectrometer | J&W DB-5MS (30 m × 0.25 mm ID, 0.25 μm film thickness) | Helium, 1.0 mL/min flow rate |
| System B | |||
| Gas chromatography | Flame ionization detector (275°C) | J&W DB-5MS (30 m × 0.25 mm ID, 0.25 μm film thickness) | Helium, 1.0 mL/min flow rate |
| System C | |||
| Ultra-performance liquid chromatography | Photodiode array detector (210–400 nm) | Waters Acquity UPLC HSS T3, (2.1 × 50 mm, 1.8 μm) | A: Water B: Acetonitrile Gradient program: A:B (70:30 for 0.5 minutes, ramp to 10:90 in 7 minutes, hold at 10:90 for 0.5 minutes, reverse to 70:30 in 1 minute, hold at 70:30 for 2 minutes) 0.50 mL/min flow rate |
| System D | |||
| Gas chromatography | Flame ionization detector (325°C) | J&W DB-5MS (30 m × 0.32 mm ID, 0.5 μm film thickness) | Helium, 2.0 mL/min flow rate |
Table A-2. Preparation and Storage of Dose Formulations in the Prenatal Developmental Toxicity Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| Preparation |
| Dose formulations of MPEP in corn oil were created by weighing an appropriate amount of lot JL44164 in a weighing container. The contents were transferred to a precalibrated glass mixing bottle. The weighing container was rinsed with corn oil vehicle (lot 0120-0576, Welch, Holme and Clark Co., Inc) into the mixing bottle to ensure complete transfer. Flasks were brought to volume with corn oil. Formulations were stirred with a spatula for ~15 minutes, mixed using a Polytron for 10–15 minutes, then stirred again for 15–30 minutes until homogenous. |
| Chemical Lot Number |
| JL44164 (AK Scientific, Inc.) |
| Maximum Storage Time |
| 42 days |
| Storage Conditions |
| Clear glass bottles with Teflon-lined caps stored at 25°C (room temperature) |
| Study Laboratory |
| Southern Research (Birmingham, AL) |
Table A-3. Results of Analyses of Dose Formulations Administered to Female Rats in the Prenatal Developmental Toxicity Study of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| Date Prepared | Date Analyzed | Target Concentration (mg/mL) | Determined Concentration (mg/mL)a | Difference from Target (%) |
|---|---|---|---|---|
| Preadministration samples | ||||
| October 11, 2016 | October 11–18, 2016 | 0.0 | BLOQ | NA |
| 31.25 | 29.8 ± 1.7 | −4.6 | ||
| 62.5 | 64.0 ± 2.8 | 2.0 | ||
| 125 | 127 ± 3.8 | 2.0 | ||
| 250 | 246 ± 18 | −1.6 | ||
| Postadministration samples | ||||
| October 11, 2016 | November 21–22, 2016 | 0.0 | BLOQ | NA |
| 31.25 | 32.1 ± 2.41 | 3.0 | ||
| 62.5 | 64.1 ± 2.42 | 3.0 | ||
| 125 | 129 ± 1.23 | 3.0 | ||
| 250 | 246 ± 6.97 | −1.6 | ||
Table A-4. Results of Analyses of Dose Formulations Administered to Female Rabbits in the Dose Range-finding Study of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| Date Prepared | Date Analyzed | Target Concentration (mg/mL) | Determined Concentration (mg/mL)a | Difference from Target (%) |
|---|---|---|---|---|
| Preadministration samples | ||||
| November 13, 2017 | November 13–14, 2017 | 0.0 | BLOQ | NA |
| 150 | 159 ± 2.20 | 6.2 | ||
| 200 | 214 ± 3.21 | 7.1 | ||
| 250 | 274 ± 3.12 | 9.8 | ||
| Postadministration samples | ||||
| November 13, 2017 | December 28–29, 2017 | 0.0 | BLOQ | NA |
| 150 | 150 ± 2.25 | −0.21 | ||
| 200 | 210 ± 0.771 | 5.2 | ||
| 250 | 303 ± 1.44 | 21.3 | ||
Table A-5. Results of Analyses of Dose Formulations Administered to Female Rabbits in the Prenatal Developmental Toxicity Study of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
| Date Prepared | Date Analyzed | Target Concentration (mg/mL) | Determined Concentration (mg/mL)a | Difference from Target (%) |
|---|---|---|---|---|
| Preadministration samples | ||||
| January 11, 2018 | January 15–16, 2018 | 0.0 | BLOQ | NA |
| 31.25 | 31.0 ± 0.188 | −0.8 | ||
| 62.5 | 61.1 ± 0.278 | −2.3 | ||
| 125 | 127 ± 0.634 | 2.0 | ||
| Postadministration samples | ||||
| January 11, 2018 | February 21–22, 2018 | 0.0 | BLOQ | NA |
| 31.25 | 32.2 ± 0.385 | 3.1 | ||
| 62.5 | 63.3 ± 0.471 | 1.3 | ||
| 125 | 132 ± 0.583 | 5.5 | ||
Figure A-1. Reference Infrared Absorption Spectrum of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
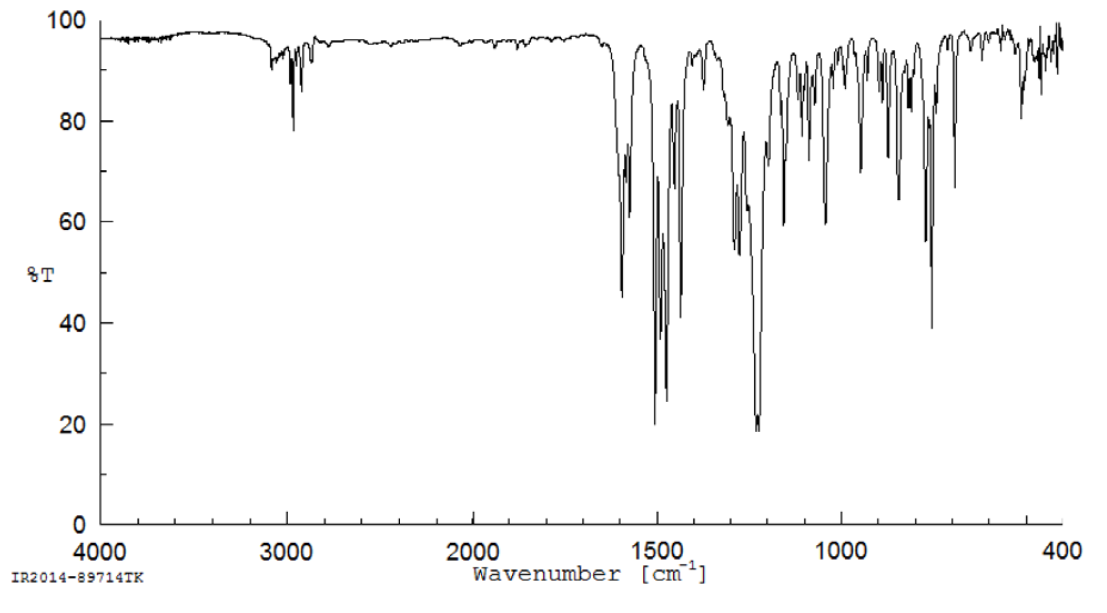
Figure A-2. 1H Nuclear Magnetic Resonance Spectrum of Reference Sample of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine
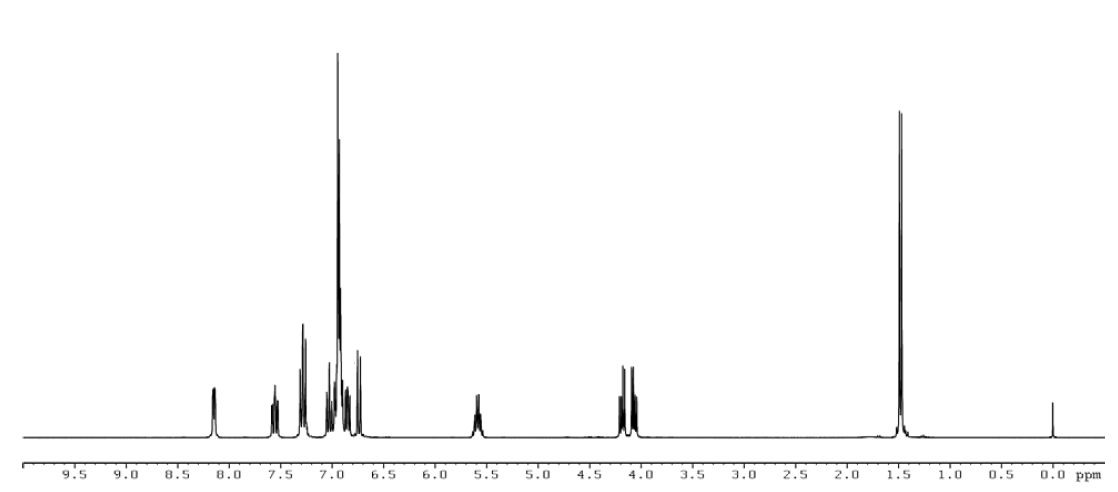
Appendix B. Ingredients, Nutrient Composition, and Contaminant Levels in Rat and Rabbit Rations
B.1.
Prenatal Developmental Toxicity Study in Rats
Table B-1. Ingredients of NIH-07 Rat Ration
| Ingredients | Percent by Weight |
|---|---|
| Ground #2 yellow shelled corn | 24.25 |
| Ground hard winter wheat | 23.00 |
| Soybean meal (47% protein) | 12.00 |
| Wheat middlings | 10.00 |
| Fish meal (62% protein) | 10.00 |
| Dried skim milk | 5.00 |
| Alfalfa meal (dehydrated, 17% protein) | 4.00 |
| Corn gluten meal (60% protein) | 3.00 |
| Soy oil (without preservatives) | 2.50 |
| Dried brewer’s yeast | 2.00 |
| Dried molasses | 1.50 |
| Calcium phosphate, dibasic (USP) | 1.25 |
| Calcium carbonate (USP) | 0.50 |
| Salt | 0.5 |
| Premixes (vitamin)a | 0.25 |
| Premixes (mineral)b | 0.15 |
| Choline chloride (70% choline) | 0.10 |
Table B-2. Vitamins and Minerals in NIH-07 Rat Rationa
| Amount | Source | |
|---|---|---|
| Vitamins | ||
| A | 6,062 IU | Stabilized vitamin A palmitate or acetate |
| D | 5,070 IU | D-activated animal sterol |
| K | 3.09 mg | Menadione (MSBC) |
| E | 22 IU | α-Tocopheryl acetate |
| Niacin | 33 mg | – |
| Folic acid | 2.4 mg | – |
| d-Pantothenic acid | 19.8 mg | d-Calcium pantothenate |
| Riboflavin | 3.8 mg | – |
| Thiamin | 11 mg | Thiamine mononitrate |
| B12 | 50 μg | – |
| Pyridoxine | 6.5 mg | Pyridozine hydrochloride |
| Biotin | 0.15 mg | d-Biotin |
| Minerals | ||
| Iron | 132 mg | Iron sulfate |
| Zinc | 18 mg | Zinc oxide |
| Manganese | 66 mg | Manganese oxide |
| Copper | 4.4 mg | Copper sulfate |
| Iodine | 1.5 mg | Calcium iodate |
| Cobalt | 0.44 mg | Cobalt carbonate |
Table B-3. Nutrient Composition of NIH-07 Rat Ration
| Nutrient | Mean ± Standard Deviation | Range | Number of Samples |
|---|---|---|---|
| Protein (% by weight) | 23.3 | – | 1 |
| Crude fat (% by weight) | 5 | – | 1 |
| Crude fiber (% by weight) | 3.49 | – | 1 |
| Ash (% by weight) | 6.6 | – | 1 |
| Amino acids (% of total diet) | |||
| Arginine | 1.376 ± 0.061 | 1.3–1.49 | 9 |
| Cystine | 0.322 ± 0.033 | 0.274–0.372 | 9 |
| Glycine | 1.147 ± 0.073 | 1.06–1.31 | 9 |
| Histidine | 0.514 ± 0.022 | 0.497–0.553 | 9 |
| Isoleucine | 0.981 ± 0.023 | 0.952–1.03 | 9 |
| Leucine | 2.011 ± 0.067 | 1.93–2.13 | 9 |
| Lysine | 1.247 ± 0.053 | 1.13–1.32 | 9 |
| Methionine | 0.486 ± 0.015 | 0.468–0.515 | 9 |
| Phenylalanine | 1.094 ± 0.021 | 1.07–1.12 | 9 |
| Threonine | 0.915 ± 0.032 | 0.883–0.961 | 9 |
| Tryptophan | 0.280 ± 0.021 | 0.265–0.326 | 9 |
| Tyrosine | 0.859 ± 0.039 | 0.785–0.894 | 9 |
| Valine | 1.131 ± 0.024 | 1.11–1.17 | 9 |
| Essential fatty acids (% of total diet) | |||
| Linoleic | 2.34 ± 0.201 | 2.04–2.59 | 9 |
| Linolenic | 0.25 ± 0.028 | 0.217–0.296 | 9 |
| Vitamins | |||
| Vitamin A (IU/kg) | 4,860 | – | 1 |
| α–Tocopherol (ppm) | 7,442 ± 22,184 | 40.3–66,600 | 9 |
| Thiaminea (ppm) | 11.1 | – | 1 |
| Riboflavin (ppm) | 14.1 ± 3.37 | 10–19.8 | 9 |
| Niacin (ppm) | 99 ± 8.63 | 87–112 | 9 |
| Pantothenic acid (ppm) | 44.8 ± 3.82 | 38.2–51.1 | 9 |
| Pyridoxinea (ppm) | 13.2 ± 3.23 | 9.63–19.7 | 9 |
| Folic acid (ppm) | 2.47 ± 0.514 | 1.68–3.09 | 9 |
| Biotin (ppm) | 0.304 ± 0.163 | 0.0–0.64 | 9 |
| Vitamin B12 (ppb) | 49.89 ± 7.06 | 41.8–61.6 | 9 |
| Choline (as chloride) (ppm) | 1,801 ± 198 | 1,570–2,200 | 9 |
| Minerals | |||
| Calcium (%) | 0.980 | – | 1 |
| Phosphorus (%) | 0.887 | – | 1 |
| Potassium (%) | 0.833 ± 0.036 | 0.769–0.88 | 9 |
| Chloride (%) | 0.641 ± 0.107 | 0.441–0.8 | 9 |
| Sodium (%) | 0.374 ± 0.047 | 0.318–0.469 | 9 |
| Magnesium (%) | 0.186 ± 0.014 | 0.170–0.218 | 9 |
| Iron (ppm) | 386.6 ± 58.0 | 276–469 | 9 |
| Manganese (ppm) | 91.58 ± 7.60 | 80.7–104 | 9 |
| Zinc (ppm) | 64.41 ± 10.64 | 52.4–89.2 | 9 |
| Copper (ppm) | 14.1 ± 2.73 | 11.9–21.1 | 9 |
| Iodine (ppm) | 1.63 ± 0.862 | 0.54–3.45 | 9 |
| Chromium (ppm) | 3.95 ± 0.030 | 3.91–4.00 | 9 |
| Cobalt (ppm) | 0.51 ± 0.282 | 0.01–0.963 | 9 |
Table B-4. Contaminant Levels in NIH-07 Rat Rationa
| Mean | Number of Samples | |
|---|---|---|
| Contaminants | ||
| Arsenic (ppm) | 0.576 | 1 |
| Cadmium (ppm) | 0.09 | 1 |
| Lead (ppm) | 0.077 | 1 |
| Mercury (ppm) | 0.018 | 1 |
| Selenium (ppm) | 0.298 | 1 |
| Aflatoxins (ppb)b | <5.00 | 1 |
| Nitrate nitrogenb,c (ppm) | <10.0 | 1 |
| Nitrite nitrogenb,c (ppm) | <0.61 | 1 |
| BHAb,d (ppm) | <1.0 | 1 |
| BHTb,d (ppm) | <1.0 | 1 |
| Aerobic plate count (CFU/g) | <10 | 1 |
| Coliform (MPN/gm) | <3 | 1 |
| Escherichia coli (MPN/g) | <10 | 1 |
| Salmonella (MPN/g) | Negative | 1 |
| Total nitrosaminese (ppb) | 4.3 | 1 |
| N–Nitrosodimethylaminee (ppb) | 1.8 | 1 |
| N–Nitrosopyrrolidinee (ppb) | 2.5 | 1 |
| Pesticides (ppm) | ||
| α-BHCb | <0.01 | 1 |
| β-BHCb | <0.02 | 1 |
| γ-BHCb | <0.01 | 1 |
| δ-BHCb | <0.01 | 1 |
| Heptachlorb | <0.01 | 1 |
| Aldrinb | <0.01 | 1 |
| Heptachlor epoxideb | <0.01 | 1 |
| DDEb | <0.01 | 1 |
| DDDb | <0.01 | 1 |
| DDTb | <0.01 | 1 |
| HCBb | <0.01 | 1 |
| Mirexb | <0.01 | 1 |
| Methoxychlorb | <0.05 | 1 |
| Dieldrinb | <0.01 | 1 |
| Endrinb | <0.01 | 1 |
| Telodrinb | <0.01 | 1 |
| Chlordaneb | <0.05 | 1 |
| Toxapheneb | <0.10 | 1 |
| Estimated PCBsb | <0.20 | 1 |
| Ronnelb | <0.01 | 1 |
| Ethionb | <0.02 | 1 |
| Trithionb | <0.05 | 1 |
| Diazinonb | <0.10 | 1 |
| Methyl chlorpyrifos | 0.015 | 1 |
| Methyl parathionb | <0.02 | 1 |
| Ethyl parathionb | <0.02 | 1 |
| Malathion | 0.11 | 1 |
| Endosulfan Ib | <0.01 | 1 |
| Endosulfan IIb | <0.01 | 1 |
| Endosulfane sulfateb | <0.03 | 1 |
B.2.
Dose Range-finding Study in Rabbits
Additional information on ingredients, vitamins, and minerals in the 5L3M rabbit ration diet can be found online.65
Table B-5. Nutrient Composition of Purina 5L3M Rabbit Ration
| Nutrient | Mean | Number of Samples |
|---|---|---|
| Protein (% by weight) | 14.9 | 1 |
| Crude fat (% by weight) | 3.4 | 1 |
| Crude fiber (% by weight) | 22.6 | 1 |
| Ash (% by weight) | 8.66 | 1 |
| Vitamins | ||
| Vitamin A (IU/kg) | 16,800 | 1 |
| Thiaminea (ppm) | 4.4 | 1 |
| Minerals | ||
| Calcium (%) | 1.36 | 1 |
| Phosphorus (%) | 0.562 | 1 |
Table B-6. Contaminant Levels in Purina 5L3M Rabbit Ration
| Mean | Number of Samples | |
|---|---|---|
| Contaminants | ||
| Arsenic (ppm) | 0.274 | 1 |
| Cadmium (ppm) | 0.15 | 1 |
| Lead (ppm) | 0.213 | 1 |
| Mercurya(ppm) | <0.0050 | 1 |
| Selenium (ppm) | 0.368 | 1 |
| Aflatoxins (ppb) | 5.3 | 1 |
| Nitrate nitrogenb (ppm) | 40 | 1 |
| Nitrite nitrogenb (ppm) | 0.122 | 1 |
| BHAb (ppm) | <1.0 | 1 |
| BHTb (ppm) | <1.0 | 1 |
| Aerobic plate count (CFU/g) | <10.0 | 1 |
| Coliform (MPN/gm) | <10.0 | 1 |
| Escherichia coli (MPN/g) | <10.0 | 1 |
| Enterobacteriaceae (CFU/g) | <10.0 | 1 |
| Total nitrosaminesc (ppb) | 49.1 | 1 |
| N–Nitrosodimethylaminec (ppb) | 7 | 1 |
| N–Nitrosopyrrolidinec (ppb) | 42.1 | 1 |
| Pesticides (ppm) | ||
| β-BHCa | <0.01 | 1 |
| γ-BHCa | <0.01 | 1 |
| δ-BHCa | <0.01 | 1 |
| Heptachlora | <0.01 | 1 |
| Aldrina | <0.01 | 1 |
| Heptachlor epoxidea | <0.01 | 1 |
| DDEa | <0.01 | 1 |
| DDDa | <0.01 | 1 |
| DDTa | <0.01 | 1 |
| Mirexa | <0.01 | 1 |
| Methoxychlora | <0.01 | 1 |
| Dieldrina | <0.01 | 1 |
| Endrina | <0.01 | 1 |
| Chlordanea | <0.01 | 1 |
| Ethiona | <0.01 | 1 |
| Diazinona | <0.01 | 1 |
| Methyl chlorpyrifosa | <0.01 | 1 |
| Methyl parathiona | <0.01 | 1 |
| Ethyl parathiona | <0.01 | 1 |
| Malathion | 0.018 | 1 |
| Endosulfan Ia | <0.02 | 1 |
| Endosulfan IIa | <0.02 | 1 |
| Endosulfane sulfatea | <0.02 | 1 |
B.3.
Prenatal Developmental Toxicity Study in Rabbits
Additional information on ingredients, vitamins, and minerals in the 5L3M rabbit ration diet can be found online.65
Table B-7. Nutrient Composition of Purina 5L3M Rabbit Ration
| Nutrient | Mean | Number of Samples |
|---|---|---|
| Protein (% by weight) | 15.1 | 1 |
| Crude fat (% by weight) | 3.5 | 1 |
| Crude fiber (% by weight) | 21.6 | 1 |
| Ash (% by weight) | 8.47 | 1 |
| Vitamins | ||
| Vitamin A (IU/kg) | 14,900 | 1 |
| Thiaminea (ppm) | 3.8 | 1 |
| Minerals | ||
| Calcium (%) | 1.240 | 1 |
| Phosphorus (%) | 0.522 | 1 |
Table B-8. Contaminant Levels in Purina 5L3M Rabbit Ration
| Mean | Number of Samples | |
|---|---|---|
| Contaminants | ||
| Arsenic (ppm) | 0.282 | 1 |
| Cadmium (ppm) | 0.151 | 1 |
| Lead (ppm) | 0.229 | 1 |
| Mercury (ppm)a | <0.0050 | 1 |
| Selenium (ppm) | 0.406 | 1 |
| Aflatoxinsa (ppb) | <5.00 | 1 |
| Nitrate nitrogenb (ppm) | 50.1 | 1 |
| Nitrite nitrogenb (ppm) | 4.3 | 1 |
| BHAa (ppm) | <1.0 | 1 |
| BHT (ppm) | 2.22 | 1 |
| Aerobic plate countc (CFU/g) | 15,000 | 1 |
| Coliformc (CFU/gm) | 10.0 | 1 |
| Escherichia colic (CFU/g) | <10 | 1 |
| Enterobacteriaceaec (CFU/g) | 30 | 1 |
| Total nitrosaminesd (ppb) | 51.9 | 1 |
| N–Nitrosodimethylamined (ppb) | 4.7 | 1 |
| N–Nitrosopyrrolidined (ppb) | 47.2 | 1 |
| Pesticides (ppm) | ||
| β-BHCa | <0.01 | 1 |
| γ-BHCa | <0.01 | 1 |
| δ-BHCa | <0.01 | 1 |
| Heptachlora | <0.01 | 1 |
| Aldrina | <0.01 | 1 |
| Heptachlor epoxidea | <0.01 | 1 |
| DDEa | <0.01 | 1 |
| DDDa | <0.01 | 1 |
| DDTa | <0.01 | 1 |
| Mirexa | <0.01 | 1 |
| Methoxychlora | <0.01 | 1 |
| Dieldrina | <0.01 | 1 |
| Endrina | <0.01 | 1 |
| Chlordanea | <0.01 | 1 |
| Ethiona | <0.01 | 1 |
| Diazinona | <0.01 | 1 |
| Methyl chlorpyrifosa | <0.01 | 1 |
| Methyl parathiona | <0.01 | 1 |
| Ethyl parathiona | <0.01 | 1 |
| Malathion | 0.011 | 1 |
| Endosulfan Ia | <0.02 | 1 |
| Endosulfan IIa | <0.02 | 1 |
| Endosulfane sulfatea | <0.02 | 1 |
Appendix C. Sentinel Animal Program
C.1.
Methods
Animals used in the National Toxicology Program are produced in optimally clean facilities to eliminate potential pathogens that might affect study results. The Sentinel Animal Program is part of the periodic monitoring of animal health that occurs during the toxicological evaluation of test compounds. Under this program, the disease state of the animals is monitored via sera or feces from extra (sentinel) or exposed animals in the study rooms. The sentinel animals and the study animals are subject to identical environmental conditions. Furthermore, the sentinel animals come from the same production source and weanling groups as the animals used for the studies of test compounds.
For these studies, blood was collected per the following methods:
-
Rats: Blood samples were collected, allowed to clot, and the serum was separated. All samples were processed appropriately with serology testing performed by IDEXX BioResearch (formerly Rodent Animal Diagnostic Laboratory [RADIL]), University of Missouri), Columbia, MO for determination of the presence of pathogens. Evaluation for endo- and ectoparasites was performed in-house by the testing laboratory.
-
Rabbits: Blood samples were collected via dried blood spot sampling technology. All samples were processed appropriately with serology testing performed by IDEXX BioResearch (formerly Rodent Animal Diagnostic Laboratory (RADIL), University of Missouri), Columbia, MO for determination of the presence of pathogens. Fur swabs were collected for ectoparasite evaluations.
The laboratory methods and agents for which testing was performed are tabulated below; the times at which samples were collected during the studies are also listed.
Table C-1. Methods and Results for Sentinel Animal Testing in Female Rats
| Collection Time Points | Arrival | End of Study |
|---|---|---|
| Number examined (females)a | 5 | 5 |
| Method/test | ||
| Multiplex fluorescent immunoassay (MFI) | ||
| Kilham rat virus (KRV) | – | – |
| Mycoplasma pulmonis | – | – |
| Pneumonia virus of mice (PVM) | – | – |
| Rat coronavirus/sialodacryoadenitis virus (RCV/SDA) | – | – |
| Rat minute virus (RMV) | – | – |
| Rat parvo virus (RPV) | – | – |
| Rat theilovirus (RTV) | – | – |
| Sendai | – | – |
| Theiler’s murine encephalomyelitis virus (TMEV) | – | – |
| Toolan’s H-1 | – | – |
| In-house evaluation | ||
| Endoparasites | – | – |
| Ectoparasites | – | – |
Table C-2. Methods and Results for Sentinel Animal Testing in Female Rabbits
| Collection Time Points | Dose Range-finding Study | Prenatal Developmental Toxicity Study |
||
|---|---|---|---|---|
| 1–2 Weeks after Study Starta | End of Study | Study Start | End of Study | |
| Number examined (females) | 4 | 9 | 1 | 10 |
| Method/test | ||||
| Multiplex fluorescent immunoassay (MFI) | ||||
| CAR bacillus | – | – | – | – |
| Clostridium piliform | – | – | – | – |
| Encephalitozoon cuniculi | – | – | – | – |
| Rotavirus | + | + | + | + |
| Immunofluorescence assay (IFA) | ||||
| Clostridium piliform | NT | NT | NT | – |
| Rotavirus | + | + | + | NT |
| Treponema | – | – | – | – |
| PCR evaluation | ||||
| Ectoparasites (fur swab) | ||||
| Myocoptes | NT | NT | NT | – |
| Radfordia/myobia | NT | NT | NT | – |
C.2.
Results
Antibodies to rotavirus were detected in all rabbit samples tested. Rotavirus is a common virus in rabbits that was not considered to have affected the study results. All other test results were negative.
Appendix D. 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine Internal Dose Assessment
D.1.
Sample Collection
D.1.1.
Prenatal Developmental Toxicity Study in Rats
On gestation day (GD) 18, blood was collected from dams designated for biological sampling from the 0 (vehicle control), 62.5, and 125 mg MPEP/kg body weight/day (mg/kg/day) groups (n = 3 or 4 per dose group). Blood was collected via cardiac puncture into tubes containing tripotassium ethylenediaminetetraacetic acid (K3 EDTA) and kept on ice until processing. Blood samples were centrifuged (refrigerated), and plasma was isolated. Following maternal blood collection, amniotic fluid was collected and pooled by litter. Fetuses were removed from amniotic sacs, euthanized by decapitation, collected, frozen, and pooled by litter. All samples were collected approximately 2 hours after the last dose and within 2 hours of each other. All samples were frozen at approximately −70°C and shipped on dry ice to the analytical laboratory at RTI International (Research Triangle Park, NC).
D.1.2.
Prenatal Developmental Toxicity Study in Rabbits
On GD 27, blood (enough to yield 1–1.5 mL plasma) was collected from does designated for biological sampling (n = 3 per dose group) at four time points (before dosing and at 4, 8, and 24 hours postdose). Collection at 24 hours occurred before dosing on GD 28. On GD 28, approximately 2 hours after the last dose, blood was collected from the same does and their fetuses. For does, blood was collected from the central ear artery or lateral ear vein into tubes containing K3 EDTA. All samples were collected approximately 2 hours after the last dose and within 2 hours of each other and kept on ice until processing.
Following maternal blood collection on GD 28, does were euthanized and fetuses were collected, individually weighed, and euthanized. Trunk blood was collected (as much as possible) from each fetus. All samples were collected approximately 2 hours after the last dose and within 2 hours of each other and kept on ice until processing.
Plasma was isolated as described above and flash frozen at approximately −70°C. Frozen samples were shipped on dry ice to the analytical laboratory at RTI International (Research Triangle Park, NC).
D.2.
Sample Analysis
D.2.1.
Method Qualification and Results
All samples were analyzed using a qualified method. The method was evaluated using commercially procured adult male Sprague Dawley rat plasma by analyzing calibration standards and quality control (QC) samples in replicates to demonstrate linearity, sensitivity, precision, and accuracy over the concentration range 10–1,000 ng/mL. Accuracy was evaluated as percent relative error (% RE) and precision as relative standard deviation (RSD). The limit of quantitation (LOQ) was evaluated by preparing six replicates at the lowest calibration standard. The limit of detection (LOD) was defined as three times the standard deviation of the LOQ response expressed as concentration. Precision and accuracy were evaluated at two concentrations (20 and 500 ng/mL) using three independent standards prepared on the same day. Selectivity was assessed by analyzing triplicate matrix blanks (adult male commercial Sprague Dawley rat plasma, GD 18 study Sprague Dawley rat dam plasma, amniotic fluid, and fetus homogenate) for background interferences and comparing to the response relative to the LOQ (10 ng/mL, Table D-2). Fetal homogenates were prepared by homogenizing fetuses in chilled water (1:1, w/v). Storage stabilities of MPEP in the study matrices were assessed at 200 ng/mL when stored at approximately −70°C; data were compared against freshly prepared samples at the same concentration.
Two standard stock solutions of MPEP were prepared at 250 and 500 ug/mL in methanol. Spiking solutions of MPEP (25–5,000 ng/mL) were prepared in methanol using alternate stock solutions and were used to prepare the matrix calibration standards and QC samples. A working solution of the internal standard d4-2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine (d4-MPEP, C/D/N Isotopes, Pointe-Claire, Quebec, Canada) was prepared at 500 ng/mL in methanol. Standard solutions were stored refrigerated (~5°C) when not in use.
Plasma calibration standards were prepared by fortifying 50 μL of blank commercial male Sprague Dawley rat plasma with 10 μL of the appropriate spiking standard to obtain plasma MPEP concentrations of 10, 20, 100, 250, 500, and 1,000 ng/mL. QC samples were prepared similarly at 20 and 500 ng/mL. For matrix blanks, 10 μL of methanol were substituted for the spiking solution. A 10 μL aliquot of the internal standard solution was added to each sample, followed by 150 μL acetonitrile. Samples were vortexed for 1 minute and centrifuged at 12,000 rpm for 10 minutes at 4°C, and the supernatants were collected. Amniotic fluid and fetal homogenate samples were prepared similarly using 50 μL aliquots.
Supernatants were analyzed by ultra-performance liquid chromatography (UPLC) with tandem mass spectrometry (MS/MS) using the system in Table D-1.
Calibration curves were generated by plotting the peak area ratios of MPEP to d4-MPEP as a function of analyte concentration. The regression model for MPEP was a linear weighted least squares algorithm with a weighting factor of 1/concentration-squared (1/x2). The concentration of MPEP was calculated using response ratio and the regression equation. Plasma and amniotic fluid data are expressed as ng/mL, whereas fetus data are expressed as ng/g fetus.
The method qualification data are given in Table D-2, and stability data are given in Table D-3. Background concentrations of MPEP were detected in all matrices and were lower than the LOQ set for the matrix concentration range (44%–50% of LOQ). These data suggest that the analytical method developed for commercial Sprague Dawley rat plasma was suitable for quantitation of MPEP in rat and rabbit study matrices. In addition, MPEP was stable in rat study matrices (GD 18 dam plasma, amniotic fluid, and fetus homogenate) under the study sample storage conditions and duration.
D.2.2.
Study Sample Analysis
Study biological samples were thawed prior to aliquoting. Study samples were prepared and analyzed similar to matrix standards above except the addition of MPEP. Fetuses were thawed, and two were randomly selected and weight recorded. Each fetus was combined with 2.3-mm stainless-steel beads equal to two times the weight of fetus and homogenized at 1,750 rpm for 2 minutes. A 1:1 volume of fetal homogenate to chilled water (1:1, w/v) was added and the fetuses were homogenized again at 1,750 rpm for 2 minutes. The two fetuses were combined and homogenized together at 1,750 rpm for 1 minute, then placed on ice until analysis. The combined fetus homogenate was then prepared using the same procedure as for plasma samples.
Study samples that were above the upper limit of the calibration curve (1,000 ng/mL) were reanalyzed by diluting 1:10 with the corresponding matrix and were processed similar to above.
Table D-1. Ultra-performance Liquid Chromatography with Tandem Mass Spectrometry System and Parameters
| Instrument Parameter | |
|---|---|
| System | Waters Acquity UPLC/Applied Biosystems 4000 QTRAP (Water Corp., Milford MA/Applied Biosystems, Framingham, MA) |
| Column | Waters Acquity UPLC BEH C18 (2.1 × 50 mm ID, 1.7 μm film thickness) |
| Mobile phase | A: 0.1% formic acid in 80:20 water:acetonitrile B: 0.1% formic acid in acetonitrile |
| Binary gradient | Hold at 80:20 for 2 minutes, ramp to 5:95 in 10 minutes, hold at 5:95 for 2 minutes, reverse to 80:20 in 0.5 minutes, hold at 80:20 for 1.5 minutes; total run time = 16 minutes |
| Flow rate | 0.3 mL/minute |
| Injection volume | 5 μL |
| Ionization mode | ESI, positive ion mode |
| Curtain gas | Nitrogen, 35 psi |
| CAD gas | 12 psi |
| IonSpray voltage | 4,000 V |
| Source temperature | 350°C |
| Ion source gas 1 | Nitrogen, 15 psi |
| Ion source gas 2 | Nitrogen, 50 psi |
| Interface heater | On |
| MRM transitions | 321.9/95.9 (MPEP); 326.1/100.1 (IS) |
| Declustering potential | 35 V (MPEP); 45 V (IS) |
| Entrance potential | 10 V |
| Collision energy | 23 V (MPEP); 35 V (IS) |
| Collision cell exit potential | 8 V (MPEP); 10 V (IS) |
| Data system | AB Sciex Analyst 1.6.2 |
Table D-2. Analytic Method Qualification for Detection and Quantitation of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine in Biological Matrices
| Parameter | Result |
|---|---|
| Plasma matrix concentration range (ng/mL) | 10–1,000 |
| LODa (ng/mL) | 3.12 |
| LOQb (ng/mL) | 10.6 |
| Precision (% RSD) (at LOQ) | 10.2 |
| Accuracy (mean % RE) (at LOQ) | ≤13.7 |
| Correlation coefficient (r) | ≥0.99 |
| Assay precision and accuracyc | |
| Commercial male Sprague Dawley rat plasma | |
| Precision (% RSD) | ≤3.6 |
| Accuracy (% RE) | ≤ ± 9.9 |
| Study GD 18 rat dam plasmad | |
| Precision (% RSD) | ≤8.1 |
| Accuracy (% RE) | ≤ ± 15.4 |
| Study GD 18 rat fetal homogenated | |
| Precision (% RSD) | ≤ 20.0 |
| Accuracy (% RE) | ≤ ± 10.3 |
| Study GD 18 rat amniotic fluidd | |
| Precision (% RSD) | ≤4.1 |
| Accuracy (% RE) | ≤ ± 7.9 |
| Rabbit doe plasma | |
| Precision (% RSD) | 14.3 |
| Accuracy (mean % RE) | 1.8 |
| Rabbit fetal plasma | |
| Precision (% RSD) | 8.03 |
| Accuracy (mean % RE) | 4.9 |
| Method selectivity (%)e | |
| Male Sprague Dawley rat plasma | 46 |
| Study GD 18 rat dam plasmad | 47 |
| Study GD 18 rat fetal homogenated | 33 |
| Study GD 18 rat amniotic fluidd | 40 |
| Rabbit doe plasma | 50 |
| Rabbit fetal plasma | 48 |
Table D-3. Stability Data for 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine in Biological Matricesa
| Matrix (Storage Duration) | Mean % of Day 0 (% RSD) |
|---|---|
| Study GD 18 rat dam plasma (95 days) | 135 (12.9) |
| Study GD 18 rat fetal homogenate (104 days) | 75.2 (6.6) |
| Study GD 18 rat amniotic fluid (95 days) | 74.7 (1.8) |
Appendix E. Exploratory Developmental Toxicity of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine in Zebrafish Embryos
Study Rationale
The embryonic zebrafish model is considered a useful alternative organism for high-throughput developmental toxicity testing66,67 or to complement results of rodent prenatal developmental toxicity studies. 2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine (MPEP) exposures during susceptible windows of development (i.e., in utero) have been hypothesized to cause or enhance the incidence of microcephaly in humans. Due to the transparency and rapid development of zebrafish embryos, adverse effects to the head or craniofacial region, such as microcephaly, can be monitored quickly and relatively easily. In previously published work, MPEP exposure to zebrafish embryos did not result in abnormal brain size.68 However, a broader screening assessment in zebrafish embryos revealed that MPEP can cause morphological alterations, including craniofacial deficits, at exposure concentrations of 5–30 μM.57,58 Inconsistent results across laboratories could be explained by different experimental designs and analysis. Due to varying results in the literature, the National Toxicology Program (NTP) elected to evaluate MPEP developmental toxicity in the zebrafish model for a comparison to prenatal toxicity data obtained in rats and rabbits.
E.1.
Materials and Methods
All experiments were conducted at Biobide (Donostia-San Sebastián, Spain). MPEP (lot number: 2-SEH-64-1, Toronto Research Chemicals, purity 99%) was supplied by the National Toxicology Program (NTP) (Research Triangle Park, NC) and studies were completed in conjunction with several other test chemicals described in Quevedo et al.69
In brief, the adult zebrafish line Tg(Cmlc2: copGFP) was used to evaluate embryo toxicity following chemical exposure. Fertilized embryos, at 4 hours postfertilization, were placed in 24-well plates with 0.5% dimethylsulfoxide (DMSO, vehicle control) or the corresponding MPEP concentration (0.5, 1, 2, 5, 10, 20, 50, or 100 μM; n = 15 embryos per concentration). The plates were covered and wrapped with aluminum foil to avoid degradation of light-sensitive compounds. Fifteen embryos were analyzed per condition after 2 or 4 days of incubation (i.e., 2 or 4 days postfertilization [dpf]) at 28.5°C. Detailed examination of embryo morphology (including malformations in the head, heart, and tail, deformed body shape, and the presence of edemas) and mortality was performed at 2 or 4 dpf. Embryo morphology was visualized under a stereo microscope (Olympus SXZ10, Waltham, MA) by experienced technicians. Morphologies found on study were recorded as present (1) or absent (0). The presence of morphological alterations was totaled for all fish to evaluate whether there was a concentration-related effect of MPEP exposure at 4 dpf. Statistical analysis was not conducted. Incidences of morphological alterations were compared to results in Quevedo et al.69 to provide context regarding the abundance of phenotypes in zebrafish.
E.2.
Results
Individual fish results are available in the NTP Chemical Effects in Biological Systems (CEBS) database.55 At 4 dpf, MPEP was overtly toxic in embryos exposed to 50 or 100 μM. In these concentration groups, 100% of fish did not survive and therefore a morphological assessment was not completed. Survival was not decreased in any other concentration group except at 20 μM MPEP, in which survival was 20% lower than for the vehicle control group. At MPEP exposure concentrations of 5–20 μM, occurrences of morphological alterations such as microcephaly, yolk sac edema, yolk opacity, heart edema, microphthalmia, and curved body were increased compared to DMSO vehicle control fish. Figure E-1 provides representative images of zebrafish larvae exposed to 5, 10, and 20 μM MPEP. Table E-1 tabulates the number of larvae that died and lists the occurrences of each type of morphological alteration up to 20 μM MPEP.
Figure E-1. Representative Images Corresponding to 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine Exposure in Zebrafish Four Days Postfertilization
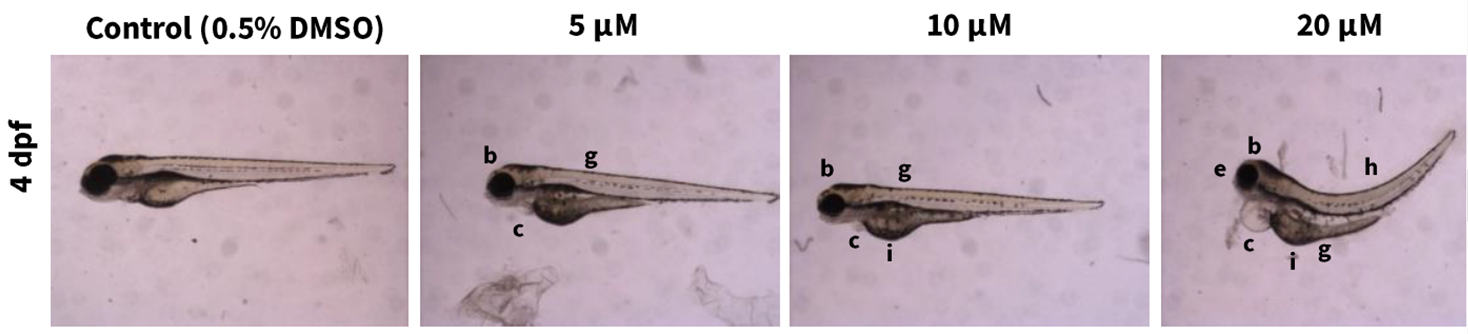
DMSO = dimethylsulfoxide; dpf = days postfertilization. Observed effects include microcephaly (b), heart edema (c), microphthalmia (e), yolk sac edema (g), curved body shape (h), and yolk opacity (i).
Table E-1. Summary of Morphological Alterations in Zebrafish Following 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine Exposure
| MPEP Concentration (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DMSO | 0.5 | 1 | 2 | 5 | 10 | 20 | 50 | 100 | |
| n | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Morphology endpoint | |||||||||
| Mortality | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 15 | 15 |
| Jaw morphology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Microcephaly or abnormal head shape | 0 | 0 | 1 | 1 | 6 | 14 | 12 | NA | NA |
| Microphthalmia/cyclopia | 0 | 0 | 1 | 0 | 0 | 2 | 2 | NA | NA |
| Head edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Malformation of the sacculi/otoliths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Heart edema/irregular shape | 0 | 0 | 1 | 2 | 2 | 6 | 10 | NA | NA |
| Abnormal heartbeat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Length alteration | 0 | 0 | 1 | 0 | 0 | 0 | 0 | NA | NA |
| Curved/curled | 0 | 0 | 0 | 0 | 0 | 2 | 10 | NA | NA |
| Notochord morphology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Tail malformations (including tail fins) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Yolk sac edema | 0 | 0 | 1 | 0 | 3 | 8 | 10 | NA | NA |
| Yolk opacity | 0 | 0 | 0 | 0 | 0 | 11 | 12 | NA | NA |
| Somite morphology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Other effects | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Hatching | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Necrotic tissues | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
E.3.
Data Interpretation
MPEP induced a number of morphological anomalies in the zebrafish head, heart, yolk sac, and body shape in a concentration-dependent manner. With the hypothesized concern for MPEP to cause craniofacial abnormalities, it was shown that MPEP does result in microcephaly with a calculated median effective concentration (EC50) of 5.4 μM. These results support findings in Padilla et al.57 and Troung et al.58 but differ from NTP studies in traditional mammalian models of developmental toxicity.
Appendix F. Supplemental Data
Tables with supplemental data can be found here: https://doi.org/10.22427/NTP-DATA-DART-07.
About This Report
Foreword
The National Toxicology Program (NTP), established in 1978, is an interagency program within the Public Health Service of the U.S. Department of Health and Human Services. Its activities are executed through a partnership of the National Institute for Occupational Safety and Health (part of the Centers for Disease Control and Prevention), the Food and Drug Administration (primarily at the National Center for Toxicological Research), and the National Institute of Environmental Health Sciences (part of the National Institutes of Health), where the program is administratively located. NTP offers a unique venue for the testing, research, and analysis of agents of concern to identify toxic and biological effects, provide information that strengthens the science base, and inform decisions by health regulatory and research agencies to safeguard public health. NTP also works to develop and apply new and improved methods and approaches that advance toxicology and better assess health effects from environmental exposures.
The NTP Technical Report series for developmental and reproductive toxicity (DART) studies began in 2019. The studies described in this NTP Technical Report series (i.e., the NTP DART Report series) are designed and conducted to characterize and evaluate the developmental or reproductive toxicity of selected substances in laboratory animals. Substances (e.g., chemicals, physical agents, and mixtures) selected for NTP reproductive and developmental studies are chosen primarily on the basis of human exposure, level of commercial production, and chemical structure. The interpretive conclusions presented in NTP DART Reports are based only on the results of these NTP studies, and extrapolation of these results to other species, including characterization of hazards and risks to humans, requires analyses beyond the intent of these reports. Selection for study per se is not an indicator of a substance’s developmental or reproductive toxicity potential.
NTP conducts its studies in compliance with its laboratory health and safety guidelines and the Food and Drug Administration Good Laboratory Practice Regulations and meets or exceeds all applicable federal, state, and local health and safety regulations. Animal care and use are in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Studies are subjected to retrospective quality assurance audits before they are presented for public review. Draft reports undergo external peer review before they are finalized and published.
The NTP DART Reports are available free of charge on the NTP website and cataloged in PubMed, a free resource developed and maintained by the National Library of Medicine (part of the National Institutes of Health). Data for these studies are included in NTP’s Chemical Effects in Biological Systems database.
For questions about the reports and studies, please email NTP or call 984-287-3211.
Collaborators
Division of the National Toxicology Program, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina, USA
Designed studies, evaluated and interpreted results, and reported findings
B.S. McIntyre, Ph.D., Study Scientist
M.V. Behl, Ph.D.
C.R. Blystone, Ph.D.
H.C. Cunny, Ph.D.
M.J. Hooth, Ph.D.
A.P. King-Herbert, D.V.M.
G.K. Roberts, Ph.D.
V.G. Robinson, M.S.
K.R. Ryan, Ph.D.
K.A. Shipkowski, Ph.D.
K.R. Shockley, Ph.D.
V.L. Sutherland, Ph.D.
S. Waidyanatha, Ph.D.
N.J. Walker, Ph.D.
Provided oversight for data management
J.M. Fostel, Ph.D.
Southern Research, Birmingham, Alabama, USA
Conducted studies and evaluated fetal findings
C.D. Hébert, Ph.D., Principal Investigator
J.R. Furr, B.S.
Conducted pilot toxicokinetic study in rats
P.B. Bushdid, Ph.D.
M.D. Merrell, Ph.D.
RTI International, Research Triangle Park, North Carolina, USA
Conducted prestart chemistry activities, dose formulations, and biological sample chemistry analyses
R.A. Fernando, Ph.D., Principal Investigator
J.C. Blake, B.A., Deputy Principal Investigator
D.B. Browning, B.S.
S.D. Cooper, M.S.
M. Silinski, Ph.D.
ASRC Federal, Research Triangle Park, North Carolina, USA
Prepared data for report
P. Brown, B.S.
C. Martini, B.S.
C. Myers, M.S.
N. Sayers, B.S.
Contributors
Division of the National Toxicology Program, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina, USA
Provided oversight for external peer review
S.L. Scruggs, Ph.D.
M.S. Wolfe, Ph.D.
Kelly Government Services, Research Triangle Park, North Carolina, USA
Supported external peer review
E.A. Maull, Ph.D. (retired from NIEHS, Research Triangle Park, North Carolina, USA)
CSS Corporation, Research Triangle Park, North Carolina, USA
Prepared quality assessment audits
S. Brecher, Ph.D., Principal Investigator
S. Iyer, B.S.
V.S. Tharakan, D.V.M.
Social & Scientific Systems, a DLH Company, Research Triangle Park, North Carolina, USA
Provided statistical analyses
S.J. McBride, Ph.D., Principal Investigator
L.J. Betz, M.S.
S.F. Harris, M.S.
J.D. Krause, Ph.D.
M.V. Smith, Ph.D.
G. Xie, Ph.D.
ICF, Fairfax, Virginia, USA
Provided contract oversight
D. Burch, M.E.M., Principal Investigator
J.C. Cleland, M.E.M.
J.A. Wignall, M.S.P.H.
Prepared and edited report
S.R. Gunnels, M.A.
T. Hamilton, M.S.
B.L. Ingle, Ph.D.
M.E. McVey, Ph.D.
K. O’Donovan, B.A.
J.R. Rochester, Ph.D.
K.A. Shipkowski, Ph.D.
S.J. Snow, Ph.D.
Supported external peer review
L.M. Green, M.P.H.
Explanation of Levels of Evidence for Developmental Toxicity
The National Toxicology Program (NTP) describes the results of individual studies of chemical agents and other test articles and notes the strength of the evidence for conclusions regarding each study. Generally, each study is confined to a single laboratory animal species, although in some instances, multiple species may be investigated under the purview of a single study report. Negative results, in which the study animals do not exhibit evidence of developmental toxicity, do not necessarily imply that a test article is not a developmental toxicant, but only that the test article is not a developmental toxicant under the specific conditions of the study. Positive results demonstrating that a test article causes developmental toxicity in laboratory animals under the conditions of the study are assumed to be relevant to humans, unless data are available that demonstrate otherwise. In addition, such positive effects should be assumed to be primary effects, unless there is clear evidence that they are secondary consequences of excessive maternal toxicity. Given that developmental events are intertwined in the reproductive process, effects on developmental toxicity may be detected in reproductive studies. Evaluation of such developmental effects should be based on the NTP Criteria for Levels of Evidence for Developmental Toxicity.
It is critical to recognize that the “levels of evidence” statements described herein describe only developmental hazard. The actual determination of risk to humans requires exposure data that are not considered in these summary statements.
Five categories of evidence of developmental toxicity are used to summarize the strength of the evidence observed in each experiment: two categories for positive results (clear evidence and some evidence); one category for uncertain findings (equivocal evidence); one category for no observable effects (no evidence); and one category for experiments that cannot be evaluated because of major design or performance flaws (inadequate study). Application of these criteria requires professional judgment by individuals with ample experience and an understanding of the animal models and study designs employed. For each study, conclusion statements are made using one of the following five categories to describe the findings. These categories refer to the strength of the evidence of the experimental results and not to potency or mechanism.
Levels of Evidence for Evaluating Developmental System Toxicity
-
Clear evidence of developmental toxicity is demonstrated by data that indicate a dose-related effect on one or more of its four elements (embryo-fetal death, structural malformations, growth retardation, or functional deficits) that is not secondary to overt maternal toxicity.
-
Some evidence of developmental toxicity is demonstrated by dose-related effects on one or more of its four elements (embryo-fetal death, structural malformations, growth retardation, or functional deficits), but where there are greater uncertainties or weaker relationships with regard to dose, severity, magnitude, incidence, persistence, and/or decreased concordance among affected endpoints.
-
Equivocal evidence of developmental toxicity is demonstrated by marginal or discordant effects on developmental parameters that may or may not be related to the test article.
-
No evidence of developmental toxicity is demonstrated by data from a study with appropriate experimental design and conduct that are interpreted as showing no biologically relevant effects on developmental parameters that are related to the test article.
-
Inadequate study of developmental toxicity is demonstrated by a study that, because of major design or performance flaws, cannot be used to determine the occurrence of developmental toxicity.
When a conclusion statement for a particular study is selected, consideration must be given to key factors that would support the selection of an individual category of evidence. Such consideration should allow for incorporation of scientific experience and current understanding of developmental toxicity studies in laboratory animals, particularly with respect to interrelationships between endpoints, impact of the change on development, relative sensitivity of endpoints, normal background incidence, and specificity of the effect. For those evaluations that may be on the borderline between two adjacent levels, some factors to consider in selecting the level of evidence of developmental toxicity are given below:
-
Increases in severity and/or prevalence (more individuals and/or more affected litters) as a function of dose generally strengthen the level of evidence, keeping in mind that the specific manifestation may be different with increasing dose. For example, malformations may be observed at a lower dose level, but higher doses may produce embryo-fetal death.
-
Effects seen in many litters may provide stronger evidence than effects confined to one or a few litters, even if the incidence within those litters is high.
-
Because of the complex relationship between maternal physiology and development, evidence for developmental toxicity may be greater for a selective effect on the embryo-fetus or pup.
-
Concordant effects (syndromic) may strengthen the evidence of developmental toxicity. Single endpoint changes by themselves may be weaker indicators of effect than concordant effects on multiple endpoints related by a common process or mechanism.
-
In order to be assigned a level of “clear evidence” the endpoint(s) evaluated should normally show a statistical increase in the deficit, or syndrome, on a litter basis.
-
In general, the more animals affected, the stronger the evidence; however, effects in a small number of animals across multiple, related endpoints should not be discounted, even in the absence of statistical significance for the individual endpoint(s). In addition, rare malformations with low incidence, when interpreted in the context of historical controls, may be biologically important.
-
Consistency of effects across generations in a multigenerational study may strengthen the level of evidence. However, if effects are observed in the F1 generation but not in the F2 generation (or the effects occur at a lesser frequency in the F2 generation), this may be due to survivor selection for resistance to the effect (i.e., if the effect is incompatible with successful reproduction, then the affected individuals will not produce offspring).
-
Transient changes (e.g., pup weight decrements, reduced ossification in fetuses) by themselves may be weaker indicators of an effect than persistent changes.
-
Uncertainty about the occurrence of developmental toxicity in one study may be lessened by effects (even if not identical) that are observed in a second species.
-
Insights from supportive studies (e.g., toxicokinetics, ADME, computational models, structure-activity relationships) and developmental findings from other in vivo animal studies (NTP or otherwise) should be drawn upon when interpreting the biological plausibility of an effect.
-
New assays and techniques need to be appropriately characterized to build confidence in their utility: their usefulness as indicators of effect is increased if they can be associated with changes in traditional endpoints.
For more information visit: https://ntp.niehs.nih.gov/go/10003.
Peer Review
The National Toxicology Program (NTP) conducted a peer review of the draft NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits by letter in April 2021 by the experts listed below. Reviewer selection and document review followed established NTP practices. The reviewers were charged to:
-
Peer review the draft Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine.
-
Comment on NTP’s interpretations of the data.
NTP carefully considered reviewer comments in finalizing this report.
Peer Reviewers
Christopher Bowman, Ph.D.
Research Fellow, Developmental and Reproductive Toxicology
Pfizer, Inc.
Groton, Connecticut, USA
Kimberley Treinen, Ph.D.
Toxicology Consultant
KT Toxicology and Drug Development LLC
Hopkinton, Massachusetts, USA
Publication Details
Publisher: National Toxicology Program
Publishing Location: Research Triangle Park, NC
ISSN: 2690-2052
DOI: https://doi.org/10.22427/NTP-DART-07
Report Series: NTP Developmental and Reproductive Toxicity Report Series
Report Series Number: 07
Official citation: National Toxicology Program (NTP). 2022. NTP developmental and reproductive toxicity technical report on the prenatal development studies of 2-((1-(4-phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) rats and New Zealand White (Hra:NZW SPF) rabbits. Research Triangle Park, NC: National Toxicology Program. DART Report 07.
Acknowledgments
This work was supported by the Intramural Research Program (ES103316, ES103318, and ES103319) at the National Institute of Environmental Health Sciences, National Institutes of Health and performed for the National Toxicology Program, Public Health Service, U.S. Department of Health and Human Services under contracts HSN273201800006C, HHSN271201800012I, HHSN273201600011C, GS00Q14OADU417 (Order No. HHSN273201600015U), HHSN273201500006C, HHSN273201400022C, HHSN273201300004C, HHSN273201300010C, and HHSN316201200054W.