Adverse Outcome Pathways
NICEATM, EPA, and other international partners are supporting the EURL ECVAM-coordinated Methods2AOP initiative. Participants in Methods2AOP are annotating assay data according to internationally accepted guidance to better relate these assays to key events in adverse outcome pathways (AOPs). The goal is to facilitate the incorporation of assays into AOP-based defined approaches for chemical safety testing, which will in turn support increased regulatory uptake of these approaches.
An article about Methods2AOP leader Clemens Wittwehr was published in the September 2022 NIEHS Environmental Factor newsletter, and a poster on Methods2AOP was presented at the 2023 meeting of the Society of Toxicology (Wittwehr et al., Alternatives to Mammalian Models II session).
An adverse outcome pathway (AOP) is a model that identifies the sequence of molecular and cellular events required to produce a toxic effect when an organism is exposed to a substance. Construction of an AOP can:
- Organize information about biological interactions and toxicity mechanisms into models that describe how exposure to a substance might cause illness or injury.
- Suggest cell- or biochemical-based tests for pathway elements that could be used to develop testing strategies for targeted toxicity.
- Identify steps in a toxicity mechanism that need improved characterization.
As described below, AOPs can provide a conceptual basis for non-animal testing strategies. NICEATM and collaborators have used the AOP framework to develop testing strategies or predictive models for:
- Embryonic vascular development
- Interaction with the androgen and estrogen receptor pathways
- Identification of potential skin sensitizers
NICEATM organized a 2014 workshop (Kleinstreuer et al. 2016) that brought together scientists from U.S. government agencies, industry, and academia to discuss how AOPs could be applied to regulatory testing.
OECD is actively supporting AOP development within its work to standardize testing methods to assess substance toxicity. OECD maintains a wiki-based interface for developing descriptions of AOPs and issues formal descriptions of well-defined AOPs. The NICEATM workshop featured demonstrations of the OECD wiki.
Elements of an Adverse Outcome Pathway
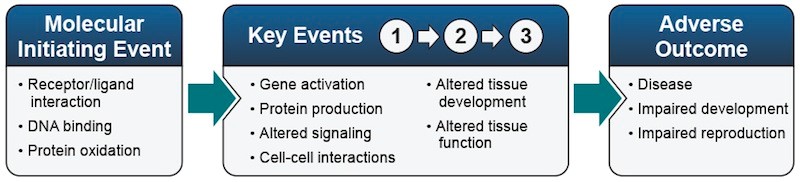
AOPs are made up of specific elements:
- A molecular initiating event is an interaction between the toxic substance and an organism, such as binding of the substance to a receptor or protein. This interaction begins the toxicity process.
- Key events after the molecular initiating event characterize the progression of the toxicity. Early key events can include changes in protein production or molecular signaling that occur in individual cells. Later key events can include altered tissue or organ function. The links between key events are described by key event relationships.
- Adverse outcomes may occur at individual or population levels. An adverse outcome for an individual organism can include disease, impaired development, or impaired reproduction. Population adverse outcomes can include changes in population structure or local extinction of a species.
Using AOPs for Research & Testing
Thousands of substances currently on the market lack full toxicity data. Traditional testing methods are too expensive and labor-intensive to fully determine the toxicity of these substances within a reasonable time frame. There is increasing interest in approaches that gather toxicity data using high-throughput cell- and biochemical-based tests. Each test is designed to assess a specific activity, such as protein binding or receptor activation, for the substance being tested. Because toxic effects cannot be predicted by any one of these high-throughput biochemical tests, it is important to test a substance using a number of tests, and then to evaluate the combined data to predict potential toxic effects. AOPs are key to combining the data generated by this approach.
Once an AOP is defined for an adverse outcome, researchers can identify specific cell- or biochemical-based tests that represent the molecular initiating events, key events, and key event relationships for that pathway. To support this goal, NICEATM is currently mapping HTS assays to endpoints such as acute systemic toxicity and developmental toxicity. To accomplish this mapping, mode-of-action terms and mechanistic targets are identified and annotated to HTS assays. This annotation is available through NICEATM’s Integrated Chemical Environment.
AOPs clarify the events and mechanisms involved in toxicity, which can help with classification and prioritization of substances for further or future testing. AOPs can be linked together by common key events to form AOP networks, which can inform on more complex toxicity endpoints such as cancer or developmental defects. The process of defining AOPs and AOP networks can also help researchers and test method developers identify areas needing improved characterization. Knowledge gaps that prevent an AOP from being fully defined indicate the need for more basic research; key events that are not represented by any suitable tests suggest future areas for test method development.
Human Pathway-based Approaches to Disease and Medicine
There is a growing recognition that, to increase the drug development success rate, a stronger focus on human-relevant data is needed. These data will support implementation of more relevant, efficient methods to understand chemical toxicity based on understanding and functionalizing human biological pathways.
A number of projects are underway internationally to mine literature, collect data, and develop AOPs for human disease. The June 2017 workshop "BioMed21: Human Pathway-based Approaches to Disease and Medicine" brought representatives from several of these projects to a single venue to identify barriers and opportunities and make recommendations regarding what is needed to achieve the goal of fully implementing a human systems-biology platform for understanding disease and improving interventions. This workshop was co-organized by NICEATM and the Human Toxicology Project Consortium. Materials from the workshop are available on the BioMed21 Collaboration website.
Pathway-based approaches are being applied to COVID-19 to better understand risk factors and effects, identify areas needing additional research, and generate hypotheses for development of treatments. The CIAO Project is a collaborative effort to organize data from research projects and facilitate collaboration across disciplines to advance knowledge about COVID-19. CIAO Project organizers presented an overview of the project in September 2021 to the NICEATM-sponsored Microphysiological Systems for COVID Research working group. NICEATM scientist Helena Hogberg is lead author on two papers (Hogberg et al. 2023; Hogberg et al. 2024) describing activities of a CIAO Project working group on neurological complications of COVID-19.