History of the Report on Carcinogens
Key Milestones
1978
Section 301(b)(4) of the Public Health Service Act, as amended, requires that the Secretary of the Department of Health and Human Services (DHHS) publish a report that identifies substances that pose a cancer hazard for people in the United States. The Report on Carcinogens (RoC) lists:
- All substances that are known to be human carcinogens or may reasonably be anticipated to be human carcinogens; and
- All substances to which a significant number of US residents are exposed.
1980
First RoC published
1993
Annual requirement for report changed to biennial
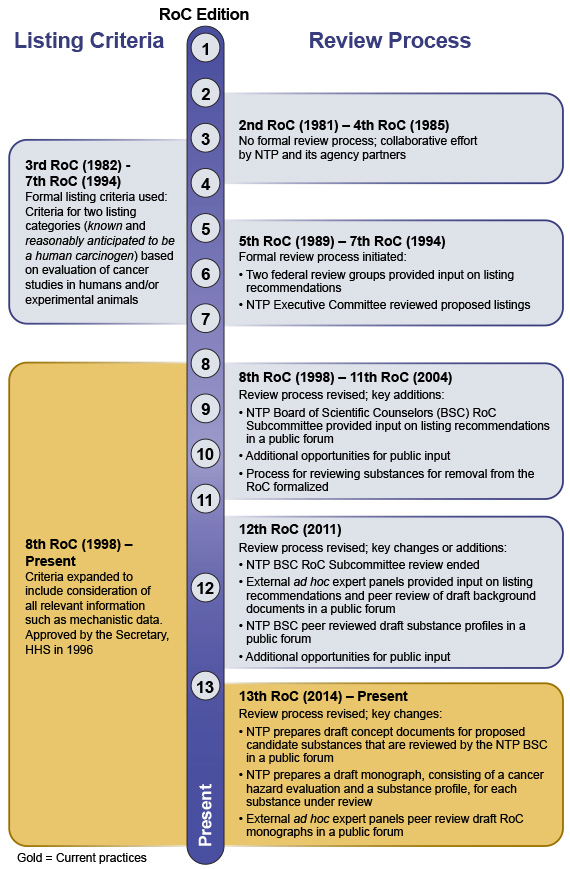
Historical Highlights of the Report on Carcinogens Criteria Listing Review Process
Since the RoC's inception in 1978, NTP has used scientifically rigorous processes and established listing criteria to evaluate substances for the RoC. The Board of Scientific Counselors (BSC) and the public have provided input leading to several evolutions of the RoC review process and listing criteria.
Pivotal Public Events
- 1987 Public Meeting
- Input on the review process for the 5th to 7th RoC review
- See schematic for key elements of that process
- 1995 Ad Hoc Working Group of the BSC Public Meeting
- Input on RoC listing criteria
- See schematic for 1996 listing criteria
- 1999 Public Meeting
- Input on RoC review process for the 10th to 11th RoC and on RoC listing criteria
- No changes were made to the RoC listing criteria
- 2004 Public Meeting
- Input on 12th RoC review process and RoC listing criteria
- No changes were made to the RoC listing criteria)
- 2011 Listening Session and NTP BSC Meeting
- Input on current RoC review process
Schematic
The following schematic describes the key elements of the RoC listing criteria and the review processes used for specific editions of the RoC. An accessible version of the schematic is also available.