Urinary System
Kidney
Narrative
The kidneys are one of the more important tissues examined. Because of its role in the filtration, metabolism, and excretion of compounds, it is often the site of test-article-induced lesions. In addition, a wide range of spontaneous renal lesions may be observed. Chronic progressive nephropathy (CPN), a spontaneous and age-related disease of rodents, may be exacerbated by chemical administration and is a confounding factor in the interpretation of renal toxicologic and carcinogenic findings.
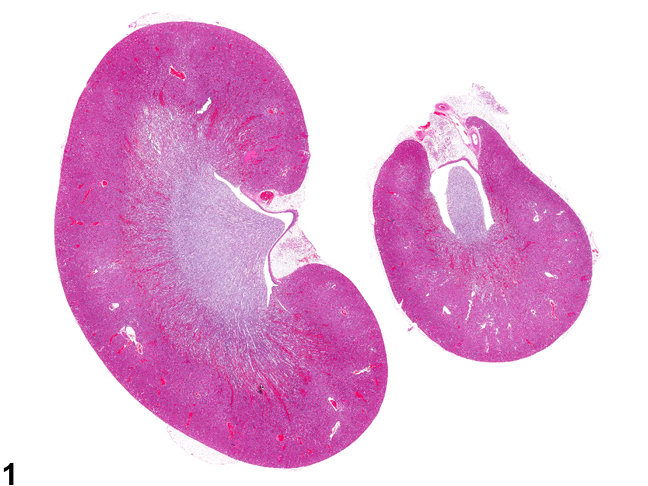
Figure 1. Longitudinally sectioned left and cross-sectioned right kidneys.
Generally for NTP studies the right kidney is cross-sectioned while the left kidney is sectioned longitudinally. It is important that both renal papillae and renal pelves are present (Figure 1). This style of sectioning helps to distinguish the kidneys. CPN and most renal toxicants generally affect both kidneys, whereas vascular, inflammatory, or proliferative lesions may affect one or both kidneys.
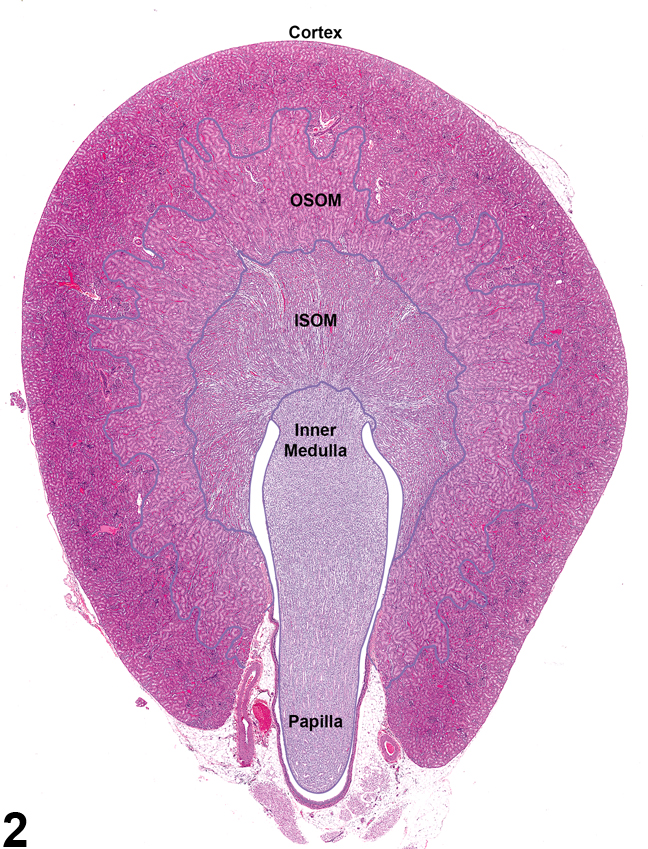
Figure 2. Demarcated regions of the kidney: cortex, outer stripe of the outer medulla (OSOM), inner stripe of the outer medulla (ISOM), inner medulla, papilla, and renal pelvis.
Kidneys contain gross landmarks: the cortex, medulla, renal papilla, and renal pelvis. Furthermore, the medulla is subdivided into indistinct landmarks: the outer stripe of the outer medulla (OSOM), the inner stripe of the outer medulla (ISOM), and the inner medulla (Figure 2).
One of the most heterogeneous tissues in the body, the kidney has a wide variety of cell types. For the most part, renal tubules and/or ducts comprise most of the renal parenchyma and are lined by specialized epithelial cells. Renal interstitial tissue is sparse in the cortex and gradually increases toward the papilla. The distribution of the renal vasculature is uniquely suited to supply more blood to the energy-active cortex.
Each area of the kidney contains defined segments of the nephron, the functional unit of the kidney, and portions of the collecting duct system. It is imperative for pathologists to understand the relationships among the anatomic locations of the various segments of the nephron or collecting duct system since some chemicals have a selective affinity for a particular nephron or duct segment.
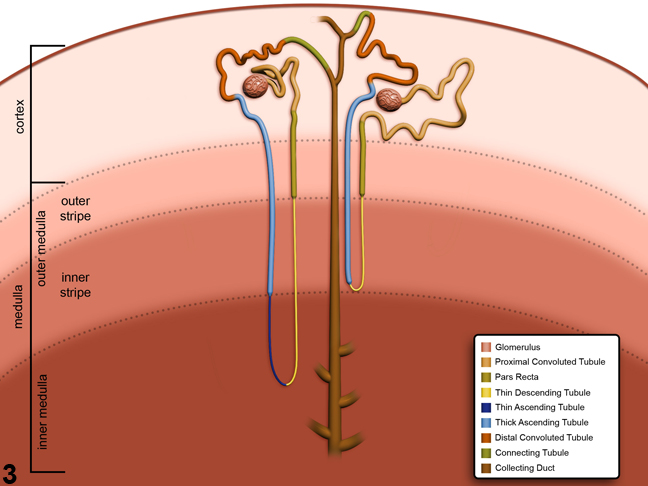
Figure 3. Schematic of the anatomic locations of the various nephron and collecting duct segments within the kidney. Image by David Sabio.
Figure 3 presents a schematic of the various segments of the nephron and collecting duct system. In general, the nephron is composed of a glomerulus, proximal convoluted tubule, pars recta (straight segment of the proximal convoluted tubule), thin descending tubule, thin ascending tubule, thick ascending tubule, and distal convoluted tubule, based on their anatomic and functional characteristics. Proximal convoluted tubules may be further subclassified as P1, P2, P3 (pars recta). Glomeruli are the filtering portion of the kidney and have a more complex structure comprising capillaries, parietal and visceral epithelium, and mesangial cells (Figure 4). Sexual dimorphism exists in the mouse kidney, where the parietal epithelium tends to be cuboidal in males but more flattened in females. Although a similar morphology has been occasionally noted in some male rats, generally rat glomeruli appear similar in males and females. Figure 5 presents tubule and glomerular basement membranes, as well the prominent luminal brush borders of proximal convoluted tubules, identified by periodic acid-Schiff staining.
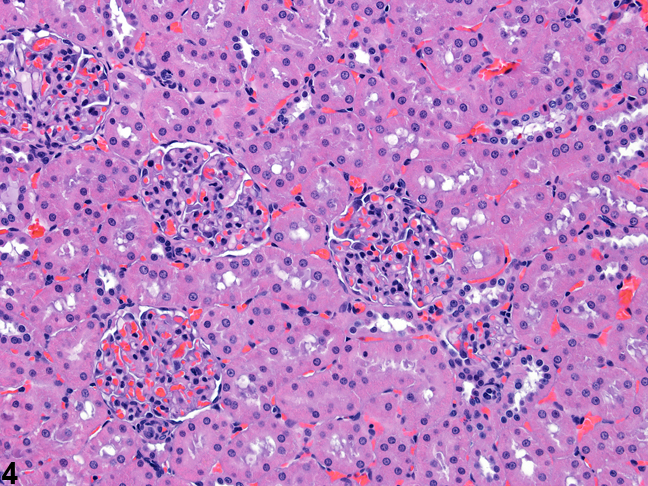
Figure 4. Histologic appearance of cortical renal tubules and glomeruli in a rat.
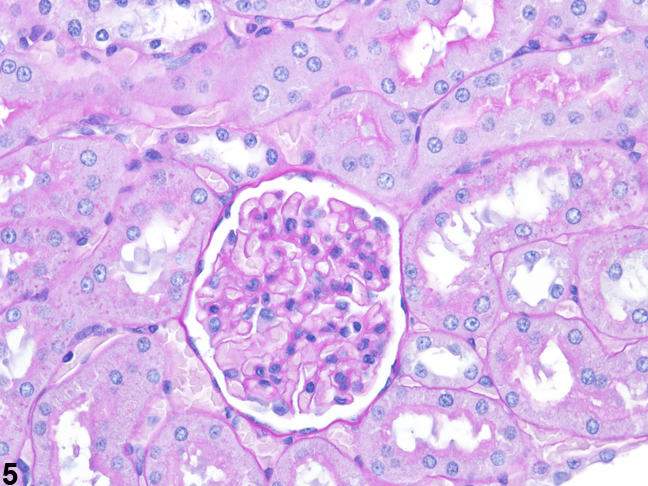
Figure 5. Tubule and glomerular basement membranes and proximal convoluted tubule brush borders outlined by PAS staining.
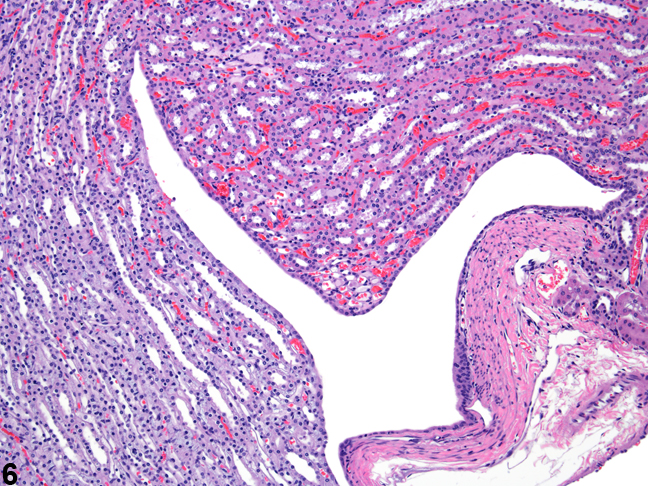
Figure 6. The fornices appear as folds within the upper portion of the renal pelves.
The upper portions of the renal pelves have specialized folds called fornices, which serve to increase the surface area of the pelvis (Figure 6). These folds may collect cellular debris and mineralized concretions as the animal ages. Figure 7 presents a section of the renal papilla, renal pelvis, and renal hilus. The larger vessels of the kidney can be seen in this section adjacent to the renal pelvis. Often islands of hematopoietic tissue may be observed in the hilus in response to increase demand. Accumulations of hematopoiesis should not be confused with inflammatory or neoplastic infiltrates. The renal pelvis is lined by urothelium.
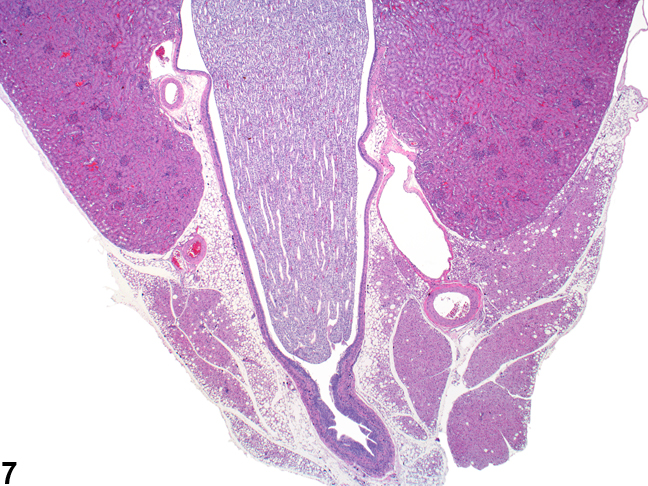
Figure 7. The papilla and surrounding renal pelvis with the adjacent renal hilar area containing the large renal artery and vein. The urothelium-lined opening to the ureter may also be seen.
Gross or microscopic developmental anomalies of the rodent kidney are uncommon, with hydronephrosis being one of the main exceptions.
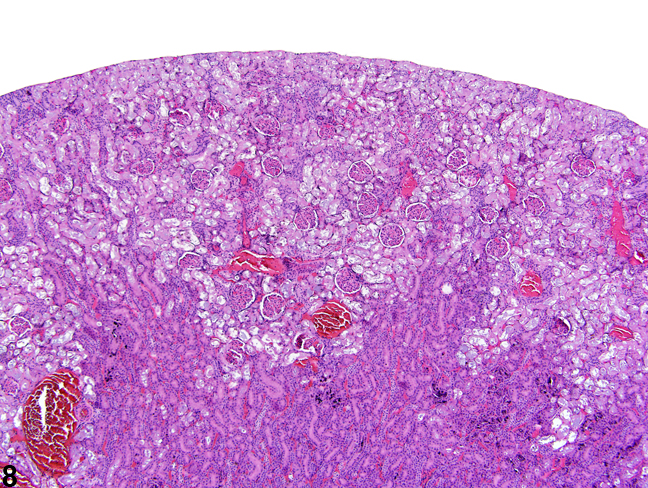
Figure 8. A low-power image of diffuse tubule autolysis mimicking necrosis.
The kidney undergoes autolysis rapidly, and kidneys from moribund animals or animals dying on test have histologic changes associated with autolysis. Even specimens immersion fixed at the time of sacrifice may contain subtle to large areas of autolysis within the kidney mimicking degeneration and necrosis (Figure 8). Autolysis must be differentiated from lesions such as tubule epithelial vacuolation, degeneration, or acute necrosis. At times this differentiation is difficult even for the seasoned pathologist. Histologic changes associated with autolysis include varying degrees of focal, zonal, or diffuse pale-staining tissue, loss of cytoplasmic and nuclear detail (rarefaction or “ghost-like” cells), retraction of tubule epithelial cells from basement membranes, and tubule vacuolation (Figure 9, Figure 10). Desquamation of tubule epithelial cells has been reported with autolysis and, therefore, is not always a reliable indicator of tubule cell necrosis.
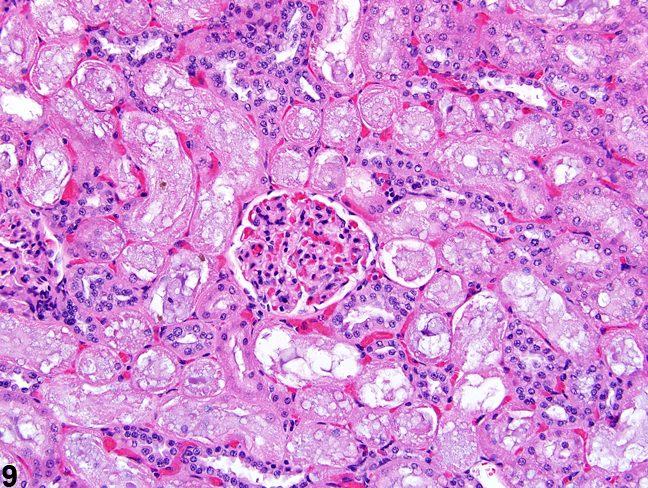
Figure 9. Consistent histologic features of autolysis are characterized by loss of cytoplasmic and nuclear detail (rarefaction) and retraction of tubule epithelial cells from basement membranes.
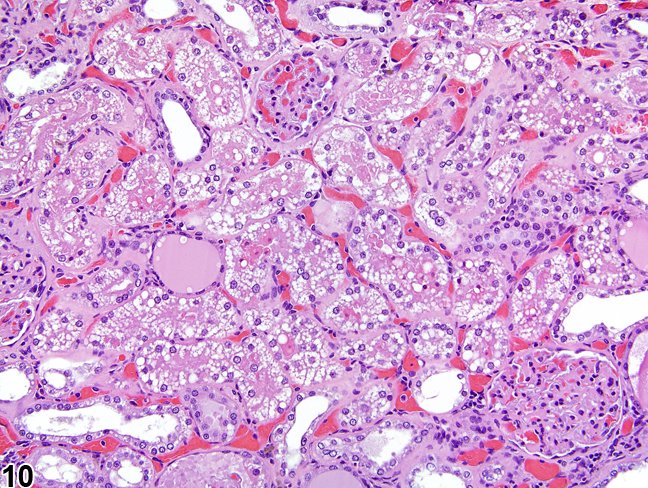
Figure 10. Autolytic vacuolation in a male rat that died on test.
Additional artifacts such as mineralization in the outer cortex may be observed and should not be confused with real mineralization (Figure 11).
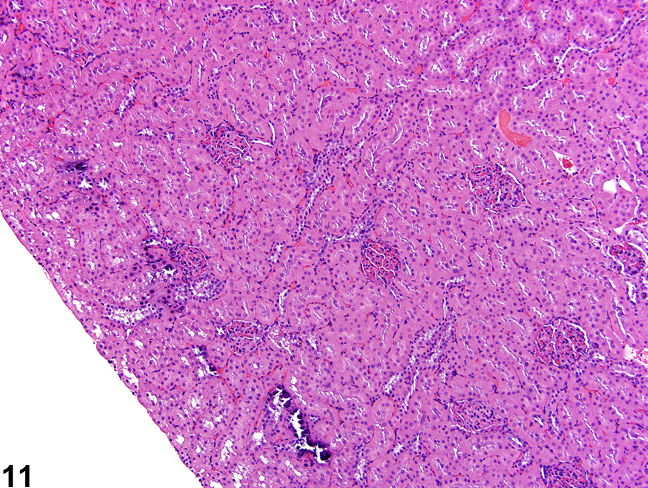
Figure 11. Artifactual mineralization represented by irregular foci of basophilia in the outer cortex of a male rat.
Calculi noted grossly in the renal pelvis may be washed out during tissue processing.
For more detailed information on the anatomy and physiology of the kidney, see Sands JM, Verlander JW. 2005. Anatomy and physiology of the kidneys. In: Toxicology of the Kidney, 3rd ed (Tarloff JB, Lash LH, eds). CRC Press, Boca Raton, FL, 3-56.
Sands JM, Verlander JW. 2005. Anatomy and physiology of the kidneys. In: Toxicology of the Kidney, 3rd ed (Tarloff JB, Lash LH, eds). CRC Press, Boca Raton, FL, 3-56.

