Cardiovascular System
Heart - Cardiomyopathy
Narrative
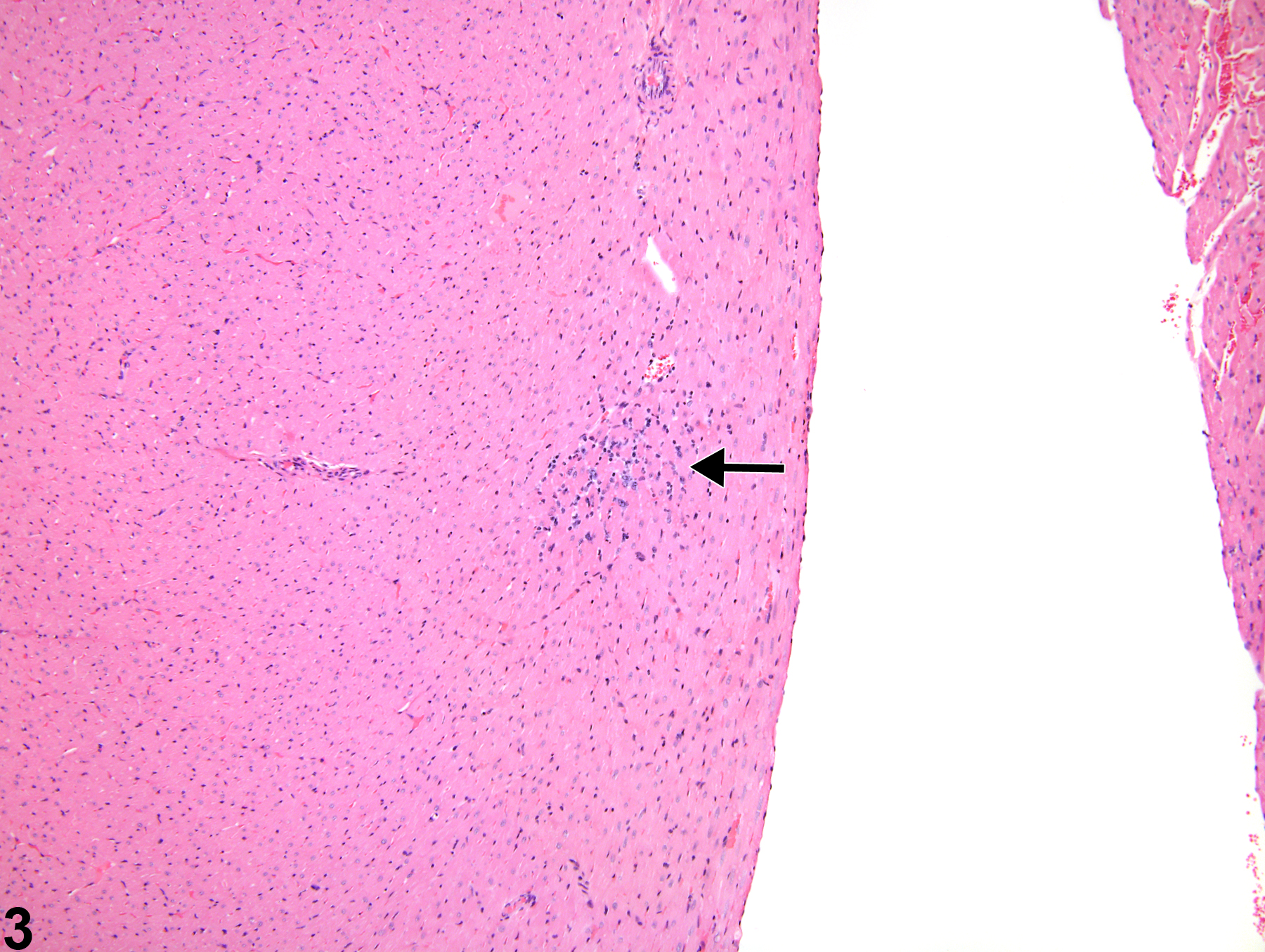
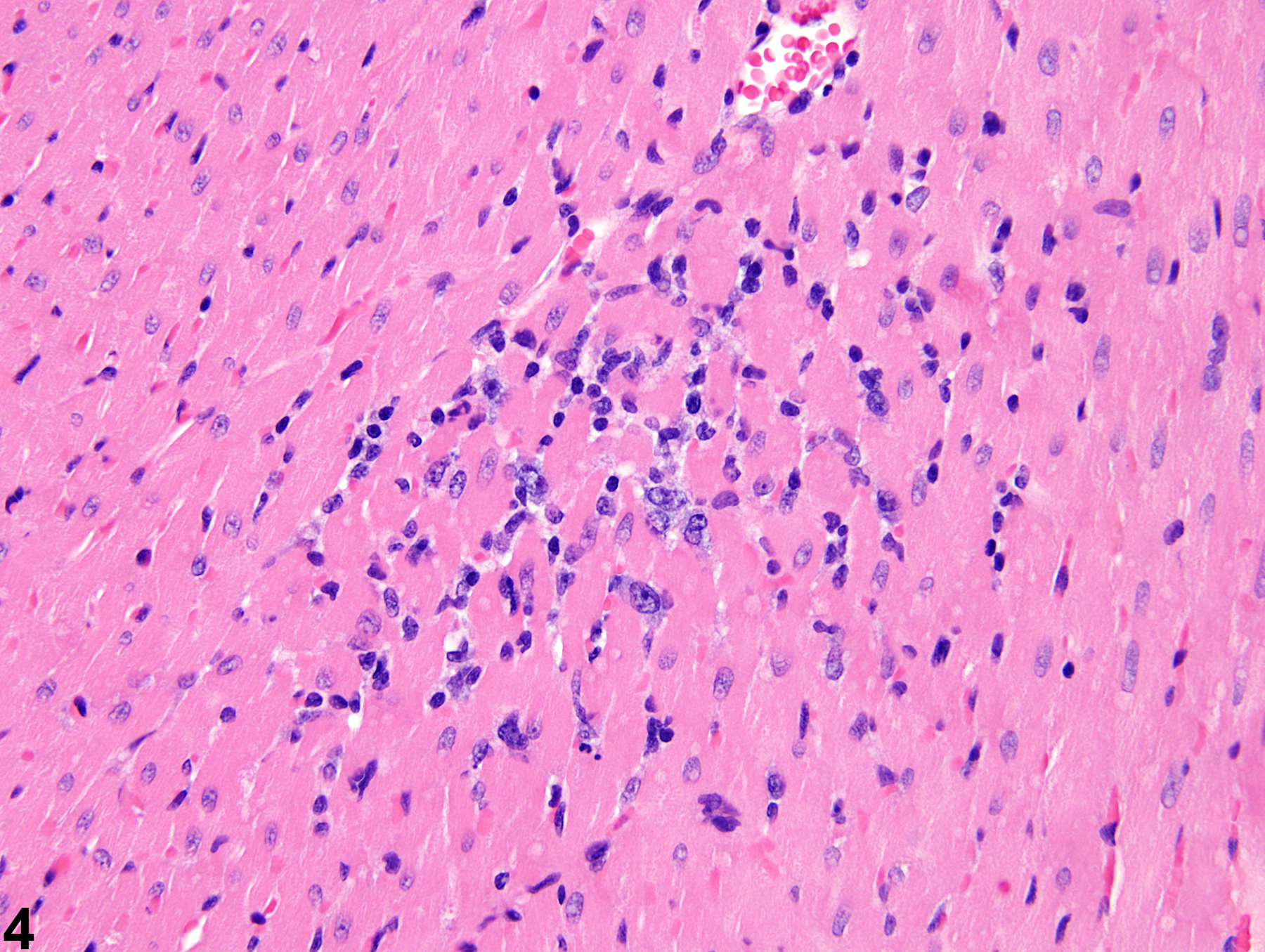
cardiomyocytes and myofiber vacuolation. These changes are often accompanied by a mixed inflammatory cell infiltrate (i.e., mononuclear and polymorphonuclear cells) that progresses to mixed mononuclear inflammatory cells only (i.e., lymphocytes and macrophages). In later stages, there is gradual replacement by fibroplasia. When cardiomyopathy is spontaneous in origin, there is time-related progression in severity (i.e., increase in size and number of foci).
Cardiomyopathy occurs more commonly in the rat than in the mouse, and in the rat more commonly in males. Lesions of cardiomyopathy are more frequently found in the left than in the right ventricle and in subendocardial than in subepicardial locations. The apex is more commonly affected than the base of the heart. The prominent inflammatory cell infiltrate seen in degenerative foci in rats is not seen in mice. Early lesions of murine progressive cardiomyopathy typically consist of multiple foci of slight increases in the interstitial cell component. Cardiomyopathy in mice appears to affect individual myocardial fibers, as affected fibers are commonly singly admixed with unaffected fibers. This appearance is in contrast to that in rats, where focal or locally extensive clusters of myocardial fibers are affected.
Myocardial cells react to toxic agents in a limited number of ways, and the basic pattern of lesion development is comparable to that seen in spontaneous cardiomyopathy (i.e., vacuolation, necrosis, inflammatory cell infiltration, and fibrosis). In addition, once lesions have healed by fibrosis, they have the same appearance regardless of whether they are spontaneous or treatment induced.
Dunnick J, Johnson JA, Horton J, Nyska A. 2004. Bis(2-chloroethoxy)methane-induced mitochondrial and myofibrillar damage: Short-term time-course study. Toxicol Sci 81:243-252.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/15201436Dunnick JK, Lieuallen W, Moyer C, Orzech D, Nyska A. 2004. Cardiac damage in rodents after exposure to bis(2-chloroethoxy)methane. Toxicol Pathol 32:309-317.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/15204972Jokinen MP, Lieuallen WG, Johnson CL, Dunnick J, Nyska A. 2005. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: The National Toxicology Program experience. Cardiovasc Toxicol 5:227-244.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/16046796Jokinen MP, Boyle M, Lieuallen WG, Johnson CL, Malarkey DE, Nyska A. 2011. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicol Pathol 39(5):850-860.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/21747121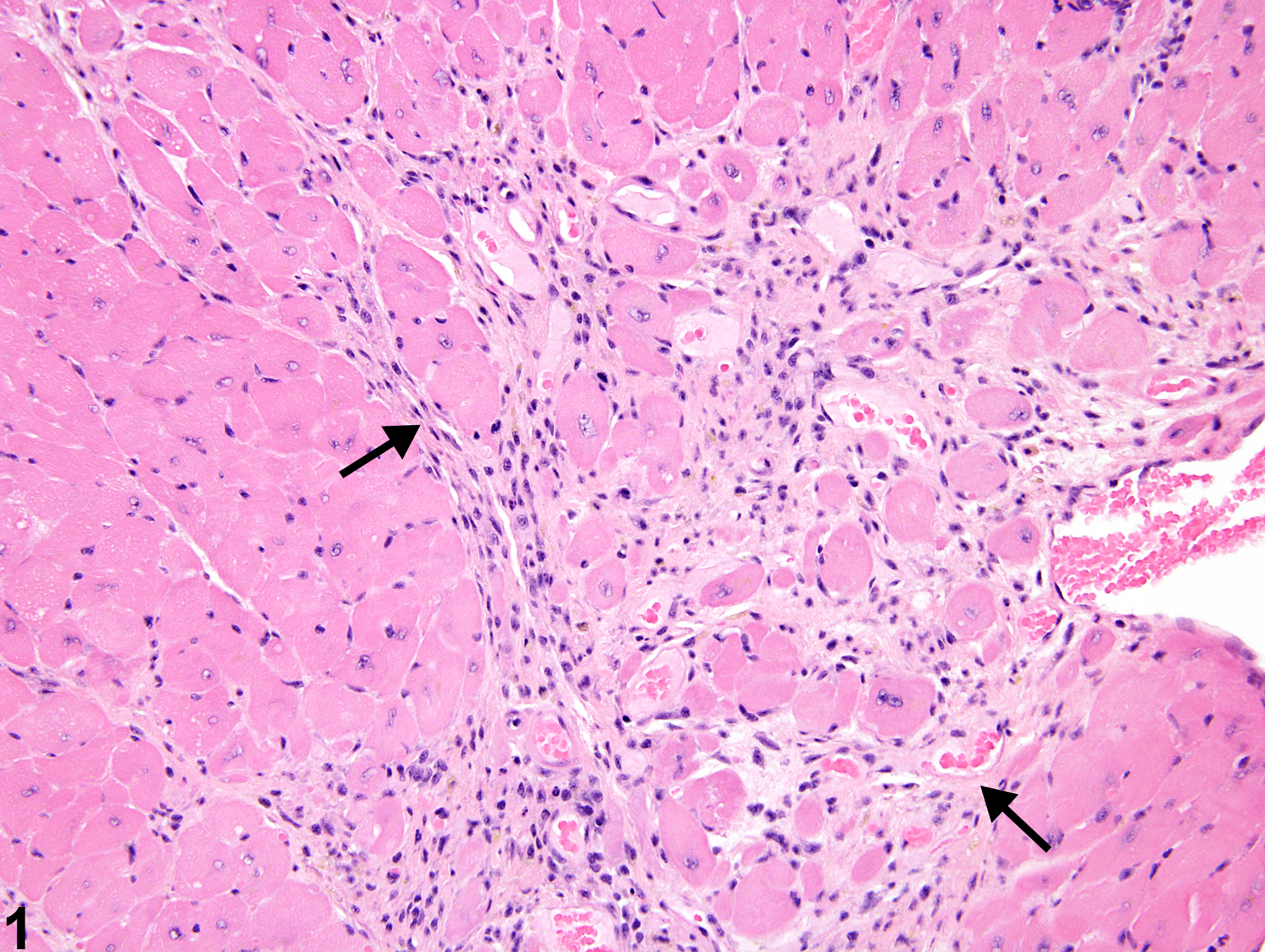
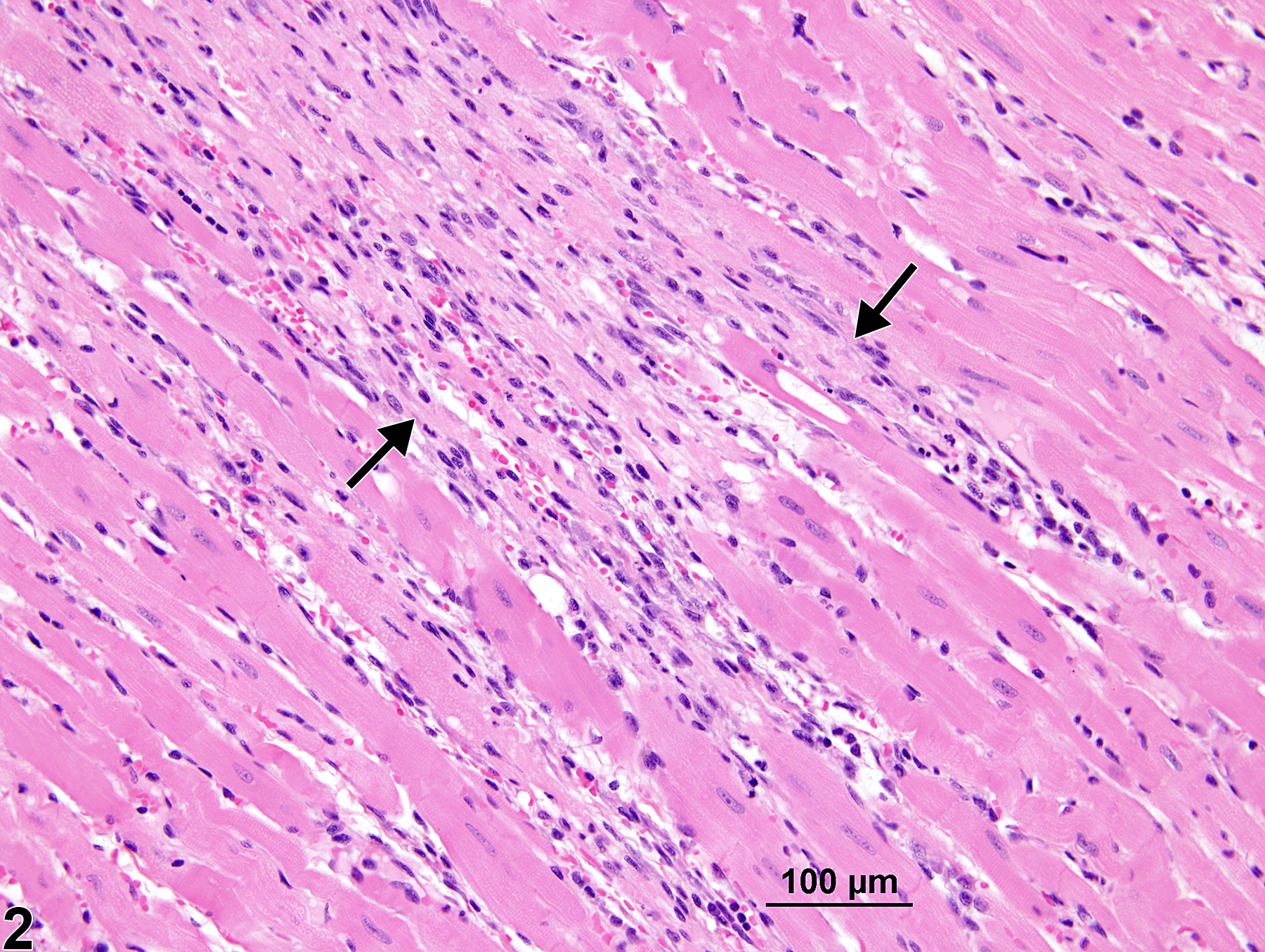
Heart - Cardiomyopathy in a male B6C3F1/N mouse from a chronic study. Branching bands of fibrous tissue and mononuclear inflammatory cells (arrows) separate cardiomyocytes.